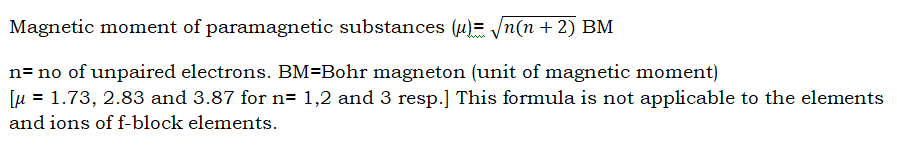Describe the discharge tube experiment
The discharge tube filled with a gas is connected to a vacuum pump in order to reduce the pressure. The electrodes are connected to a source of high voltage of 10000 volts.
- Under normal pressure (1 atm), nothing is observed.
- At 10-2 atm, the gas is found to emit light and the colour of the light depends upon the nature of the gas.
- At 10-4 atm, the emission of light ceases but the wall of the discharge tube opposite to the cathode starts glowing with a faint greenish light called fluorescence. This is due to the bombardment on the wall by cathode rays.
Mention some properties of cathode rays
- It travels in a straight line, can caste shadow, consists of negatively charged particles, and can exert mechanical pressure.
- It can produce a heating effect, can ionize the gas through which they pass, can produce X-rays when striking on the surface of hard metals like tungsten, copper, molybdenum, etc.
- It is deflected by electric and magnetic field and can pass through thin foils of metals like Al.
- It affects the photographic plate. This is called fogging.
- The e/m ratio of the particles constituting the cathode rays does not depend on the nature of the gas or the material of the cathode.
Mention some properties of anode rays
- It travels in a straight line and can caste shadow.
- It can exert mechanical pressure and is deflected by electric and magnetic field.
- The anode rays are formed by the expulsion of electrons from the atoms of the gas in the space between anode and cathode.
- The e/m ratio of the particles constituting the anode rays depends on the nature of the gas.
Mention the observations of Rutherford’s Experiment
- Most of the a- particles passed through the foil without any deflection from their path.
- A few of them were deflected at some angles.
- Very few (1 in 10000) turned back on their original path.
Mention the conclusions of Rutherford’s experiment
- Most part of the atom is empty.
- The entire mass of the atom is concentrated in a small area that carries positive charge.
- Electrons revolve around the nucleus at a large distance from it.
Different types of atomic species
Isotopes:
Atoms of the same elements with different mass numbers but the same atomic number are called isotopes. Ex: 1H1, 1H2, 1H3.
Isobars:
Atoms of different elements which possess same mass number but a different atomic number. Ex: 18Ar40 and 20Ca40, 1H3 and 2He3, 7N14and 8O17.
Isotones:
Atoms of different elements which possess different atomic and mass number but same number of neutrons. Ex: 6C13 and 7N14, 4Be9 and 5B10.
Isodiaphers:
Nuclides which have different atomic and mass number but same difference between neutron and proton numbers. Ex: 90Th 234 and 92U238
Mirror nuclei:
Nuclei having the same mass number but with the proton and neutron number interchanged. Ex : 1H3 and 2He3, 3Li7 and 4Be7.
Isoelectronic species:
Species having the same number of electrons. Ex: N3-, O2-, F–, Ne.
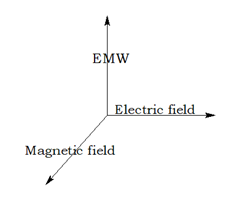
Electromagnetic Radiation
The invisible radiation caused by the combined effect of electric and magnetic field.

| TERM | DEFINITION | UNIT |
| Wavelength(λ) | Distance between any two consecutive crests or trough. | pm, Ǻ , nm |
| Frequency (ν) | Number of waves passing through a point per sec. | Hz |
| Velocity(v) | Linear distance travelled by the wave in one sec. | m/s |
| Amplitude (a) | Max height of crest or trough | cm, m |
| Wave number | Number of waves per unit length | m-1 , cm-1 |

![Structure Of Atom|Class-11 5 ALL ABOUT CHEMISTRY]() Photoelectric effect:
Photoelectric effect:
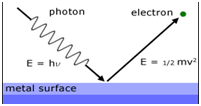
The phenomenon of ejection of electrons from the metal surface when light of suitable frequency strikes the metal. The electrons ejected are called photo-electrons.
hν= hν0 + ½ mv2
hν = Energy of the incident ray
hν0= Threshold energy= work function = Ionisation potential
ν0= Threshold frequency
½ mv2= kinetic energy of the emitted electrons
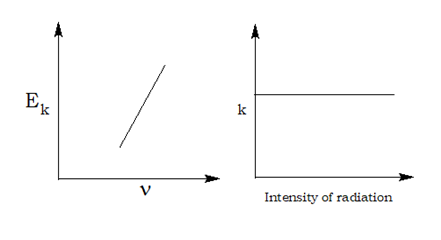
Planck’s Quantum Theory:
- Radiant energy is not emitted or absorbed continuously; rather it is absorbed or emitted by a body discontinuously in the form of small packets of energy known as quanta. For light one quantum of energy is called photon.
- The amount of energy associated with a quantum of radiation is directly proportional to its frequency. E α ν or E = hν
- A body can emit or absorb energy only in terms of integral multiples of quantum.E = nhν
Different types of spectrum
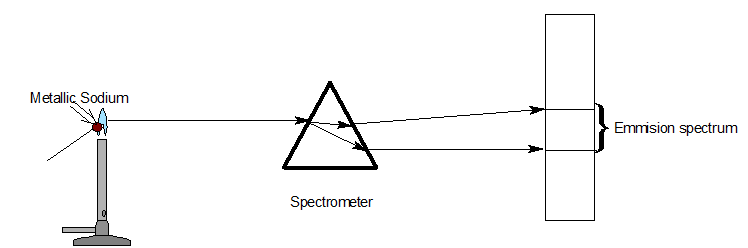
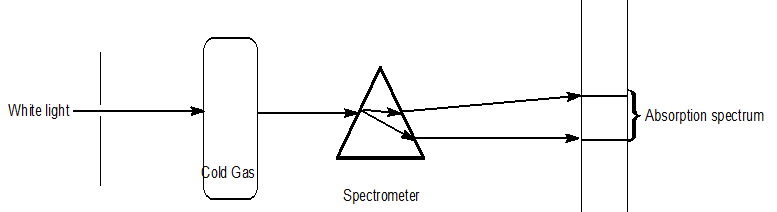
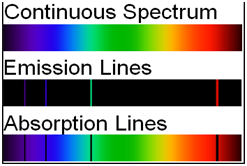
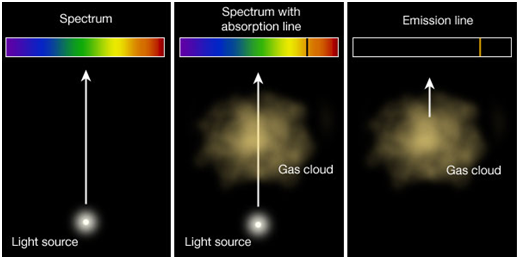
| Emission spectra | Absorption spectra |
| The spectrum of radiation emitted by a substance in excited state. | When a beam of continuous light is passed through a solution or vapour of a chemical substance, the spectrum obtained contains a number of dark lines due to the absorption of certain wavelengths. |
| It contains bright coloured lines which are separated by dark lines. | It contains dark lines at the place where coloured lines are obtained. |
| It may be continuous or discontinuous. | It is always discontinuous . |
Mention the defects of Rutherford’s Model:
- According to Classical electromagnetic theory of Maxwell, whenever a charged body revolves it gives out radiations and loses energy. Thus electron which carries negative charge, and revolves around the nucleus should continuously emit radiations and loses energy. As a result, the electron follows a spiral path and collides with the nucleus.
- When gases under pressure are subjected to electric discharge, line spectrum is produced. This cannot be explained by Rutherford.
Hydrogen spectra:
| n1 | n2 | Series | Region |
| 1 | 2,3,4,5,6,7 | Lymann | Ultra violet |
| 2 | 3,4,5,6,7 | Balmer | Visible region |
| 3 | 4,5,6,7 | Paschen | Infra red |
| 4 | 5,6,7 | Bracket | Infra red |
| 5 | 6,7 | Pfund | Infra red |
| 6 | 7 | Humphries | Infra red |

Mention the main postulates of Bohr’s Theory
- The electrons move around the nucleus in certain specifically permitted circular orbits called energy levels or energy states. An electron in a particular energy level is associated with a definite amount of energy. While moving in a particular level, it neither absorbs nor loses energy.
- Only those energy levels are permitted in which the angular momentum of an electron is an integral multiple of h/2Π(The angular momentum are quantized)
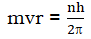
- The electron absorbs energy when it jumps from lower to higher energy levels and emits energy when it moves from higher to lower levels. The energy gained or lost is equal to the difference between the energies of the two transitions.
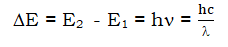
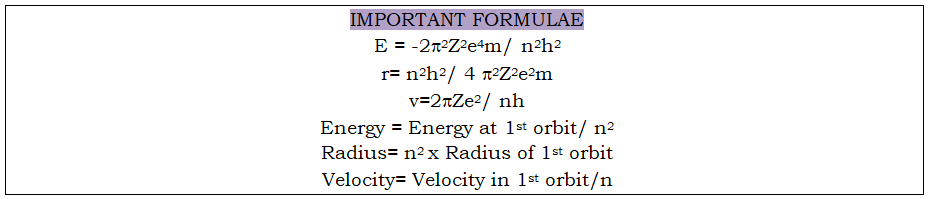
Mention the significance of negative sign of energy:
- The energy of the electron in the atom is less than the energy of a free electron and thus the atom is a stable entity.
- Electron is attracted by the nucleus.
- To remove an electron, energy must be supplied from outside.
Mention the merits and demerits of Bohr’s Theory
| Merits | Demerits |
| 1) It explains the stability of atom. | 1) It is applicable only for one electron system. |
| 2) It provides the idea of quantized energy states. | 2) It cannot explain fine spectrum |
| 3) It can explain the occurrence of lines of hydrogen spectrum. | 3) It fails to explain Zeeman effect and Stark effect. |
| 4) It provides the idea of ionization potential and resonance potential (energy required to raise an electron from its lowest energy level to any other higher level in gaseous state) | 4) It goes against Heisenberg Uncertainty principle. |
| 5) No justification to assume mvr= nh/2Π | 5) It is two dimensional but, in reality atom is three dimensional. |
Difference between Rutherford’s and Bohr’s concept:
| Rutherford’s Concept | Bohr’s Concept |
| 1) No rules on the electron | 1) Selected orbits for electron |
| 2) Electrons radiate out energy and ultimately collide with nucleus. | 2) Electrons doesn’t absorb or emit radiation of energies as long as it remain in a particular shell. |
| 3) Cannot explain line spectrum | 3) Can explain line spectrum |
De- Broglie Equation:
De- Broglie suggested that electrons shows dual nature of particles (mass) and wave ( wave length).
From Planck’s Equation, E = hc/λ
From Einstein equation, E=mc2
λ=h/mv
Where λ is the wave length
h is Planck’s Constant= 6.626 x 10-27 erg sec
m and v are the mass and velocity respectively
Heisenberg’s Uncertainty Principle:
It states that it is impossible to measure simultaneously the position and exact velocity of a sub-atomic particle.
∆x.∆v≥ h/ 4Πm
∆x= Uncertainty in position
∆v = Uncertainty in velocity
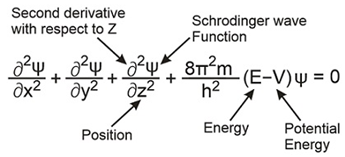
Schrodinger’s Wave Equation:
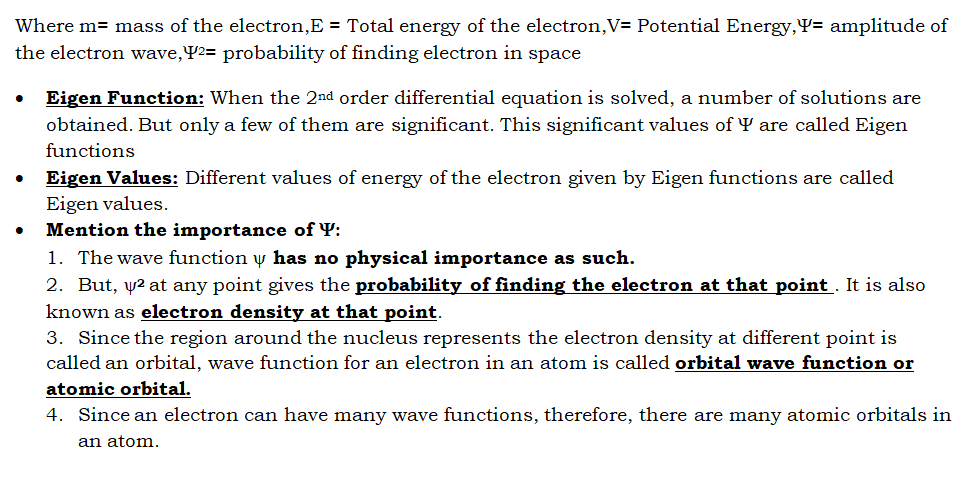
Quantum numbers:
Set of four numbers which can depict the shape, location, energy, orientation etc of the electrons.
Principal Quantum Number(n):
- It represents the number of shells
- It can have values like 1,2,3,4….
- Determines the energy of the shell
- Provides the maximum number of electrons a shell can hold(2n2)
Azimuthal Quantum Number(l):
- The line spectrum when highly resolved shows that each such line is made up of finer lines called the fine spectrum. These fine lines are due to the elliptical orbits.
- The angular momentum of a moving electron in an elliptical path is
- The minimum value of l is 0 and the maximum is n-1. Spectroscopic terms-(s- Sharp,p- Principal,d-Diffuse,f- fundamental)
- The penetrating power and screening effect of an elliptical orbital is: s> p>d>f
- The order of energy: s< p< d <f
![Structure Of Atom|Class-11 19 ALL ABOUT CHEMISTRY]() Magnetic Quantum Number(m):
Magnetic Quantum Number(m):
- The splitting of fine lines under the magnetic field is called the Zeeman effect.
- Under the influence of the external magnetic field, the electrons present in the subshells orient themselves in certain regions of space called orbitals.
- The value of m is –L to +L including zero.
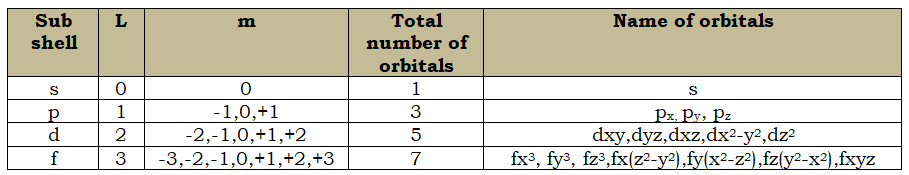
- The negative values of the magnetic quantum numbers signify that these orbitals are inclined in the direction opposite to the magnetic field and positive value indicates that these orbitals are inclined in the direction of the magnetic field.
Orbitals:
The three dimensional region or space around the nucleus of an atom where there is maximum probability of finding an electron having certain energy.
| Orbit | Orbitals |
| 1) Definite path followed by an electron | 1) Space around the nucleus where the probability of finding the electron is maximum. |
| 2)Two orbits in an atom cannot have the same energy | 2) Two orbitals can have the same energy |
| 3) Have two dimensional representation | 3) Have three dimensional representation |
| 4) Circular or elliptical in shape | 4) spherical , dumble shaped |
| 5) Can accommodate 2n2 electrons | 5) Cannot accommodate more than two electrons |
| 6) Regarded as path of electron having particular character | 6) Regarded as a negatively charged electron cloud. |
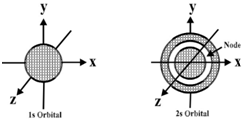
s-Orbital:
- 1s, 2s and 3s orbitals are all spherical shaped.
- Size and energy of the orbitals -1s<2s<3s
- Nodes are the region where the probability of getting the electrons is zero.

![Structure Of Atom|Class-11 23 ALL ABOUT CHEMISTRY]() p-Orbital:
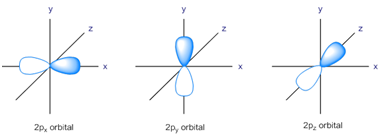
p-Orbital:
- p-orbitals are dumble shaped.
- Each p-orbitals possess two lobes, where the probability of getting the electron is max.
In between the lobes, a plane is obtained where the probability of getting the electron is zero.
- All the 3 orbitals possess the same energy and thus are degenerate.
| Radial nodes | Angular nodes |
| Radial nodes are nodes which are areas of zero probability of finding an electron and radiate like circles away from the nucleus. | Angular nodes can be visualized on boundary surface diagrams as planar nodes that separate the different signs of the orbital. |
| Spherical in nature | Flat or cones in nature |
| Radial nodes=n-L-1 | Angular nodes=L |
Total no of nodes= Radial + angular nodes = n-1
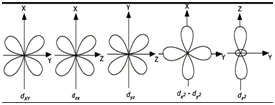
d-Orbitals:
- d-orbitals are double dumble shaped with four lobes.
- dz2 has two lobes. The annular portion orbital is called doughnut or belly band.
- All the 5 orbitals are degenerate.
Spin Quantum Number(s):
- It expresses the two opposite types of spinning motions of each electron.
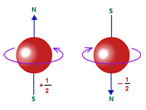
- Its values are +1/2 and -1/2
The angular momentum associated with the spinning of electrons is
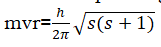
- It signifies the mode of electron spin- clockwise or anticlockwise.
- It is the only quantum no which is not obtained from Schrodinger’s wave equation.
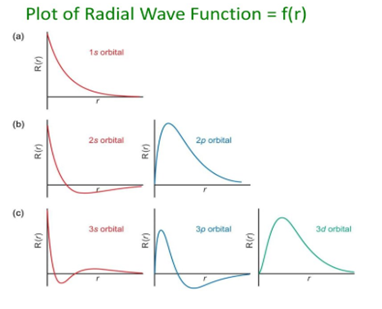
Some important graphs:
- Variation of radial part of wave function with distance from the nucleus.
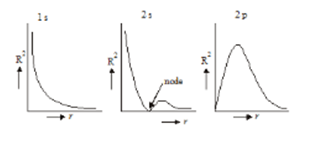
- Variation of the square of radial wave function(radial probability density) with distance from the nucleus:
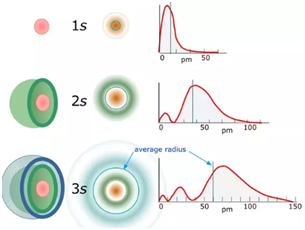
- Variation of radial probability distribution function with distance from the nucleus:

Pauli’s Exclusion Principle:
In an atom , no two electrons can have four identical quantum numbers.
Rules for filling up of orbitals:
1.Aufbau Principle:
The orbitals are filled up with electrons in the increasing order of their energy.
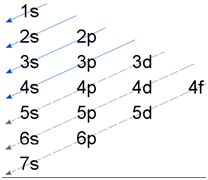
2. (n+l) rule:
The electron comes in the vacant sub-shell with lowest value of (n+l). If the value of (n+l) is same, then electron enters the sub shell having lowest value of n.
Lanthanides and actinides are exception to the (n+l) rule.
3. Hund’s Multiplicity Rule:
- Electrons continue to enter different orbitals as long as possible. Electron pairing in any orbital cannot take place until each orbital of the same sub-shell contains 1 electron.
- Two or more electrons each occupying different orbitals must have parallel spins. (Same direction of spin ↑ or ↓).
- Half filled and full filled subshells possess maximum stability. i.e., s1 , s2 , p3 ,p6 ,d5 , d10, f7 , f14 are stable configurations.
Why half-filled and full filled subshells possess maximum stability?
Symmetrical distribution of electrons:
Subshells with half filled or completely filled electrons are found to have a more symmetrical distribution of electrons. Consequently, they have less energy and more stability.
Exchange energy:
The electrons occupying degenerate orbitals can exchange their positions with other electrons with the same spin. In this process, exchange energy is released. Greater the exchange energy, more stable the configuration is.
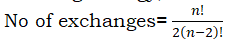
| Element | Electronic configuration according to Aufbau’s principle | Actual Electronic configuration |
| Cr(24) | [Ar]3d44s2 | [Ar]3d54s1 |
| Mo(42) | [Kr]4d45s2 | [Kr]4d55s1 |
| Cu(29) | [Ar]3d94s2 | [Ar]3d104s1 |
| Ag(47) | [Kr]4d95s2 | [Kr]4d105s1 |
| Au(79) | [Xe]4f145d96s2 | [Xe]4f145d106s1 |
| Pd(46) | [Kr]4d85s2 | [Kr]4d105s0 |
Elements with exceptional E.C-Cr(24), Cu(29), Nb(41), Mo(42),Ru(44), Rh(45), Pd(46), Ag(47), La(57), Ce(58), Gd(64), Pt(78), Au(79)
Magnetic nature of atoms and ions:
- Atoms with at least one unpaired electron are paramagnetic in nature( attracted by magnet).
- Atoms with no unpaired electron(s) are diamagnetic in nature(repelled by magnet).
- More the number of unpaired electrons, more the magnetic moment.