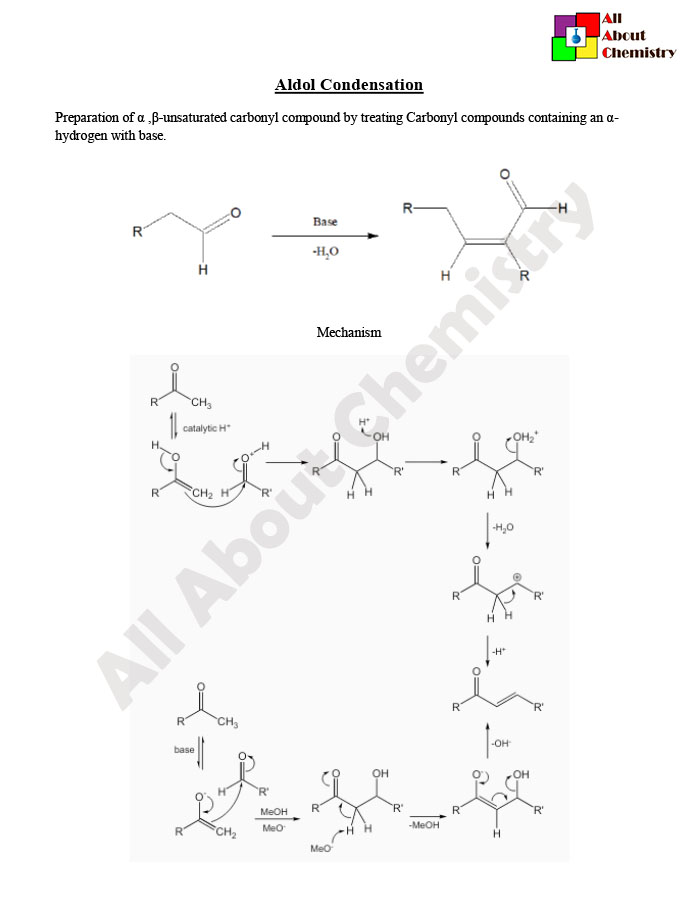
The Aldol Condensation is a versatile organic reaction that involves the formation of a new carbon-carbon bond between the alpha carbon of one carbonyl compound (aldehyde or ketone) and the carbonyl carbon of another, resulting in the formation of a beta-hydroxy carbonyl compound (aldol). This reaction can occur under both acidic and basic conditions.
Here’s the general reaction scheme:
Carbonyl Compound 1+Carbonyl Compound 2→β-Hydroxy Carbonyl Compound (Aldol)
The mechanism of the Aldol Condensation can vary depending on whether the reaction is carried out under acidic or basic conditions. However, in both cases, the key steps involve enolate formation, nucleophilic attack, and subsequent protonation.
Under basic conditions:
- Enolate Formation: The base deprotonates one of the carbonyl compounds, typically at the alpha carbon, generating an enolate ion.
- Nucleophilic Attack: The enolate ion acts as a nucleophile and attacks the carbonyl carbon of another carbonyl compound, forming a carbon-carbon bond. This step results in the formation of a beta-hydroxy carbonyl compound.
- Proton Transfer: Proton transfer occurs to stabilize the product, resulting in the formation of the aldol compound.
Under acidic conditions:
- Protonation: The carbonyl compound is protonated by an acid catalyst, typically at the oxygen atom.
- Carbocation Formation: The protonated carbonyl compound undergoes keto-enol tautomerization to form an enol intermediate.
- Nucleophilic Attack: The enol intermediate acts as a nucleophile and attacks the carbonyl carbon of another carbonyl compound, forming a carbon-carbon bond. This step results in the formation of a beta-hydroxy carbonyl compound.
- Proton Transfer: Proton transfer occurs to stabilize the product, resulting in the formation of the aldol compound.
The Aldol Condensation reaction finds numerous applications in organic synthesis due to its ability to form carbon-carbon bonds and create complex molecules. Some of the key applications include:
- Synthesis of β-Hydroxy Carbonyl Compounds (Aldols): The primary application of the Aldol Condensation is in the synthesis of β-hydroxy carbonyl compounds, also known as aldols. These compounds serve as versatile intermediates for further functionalization to yield a wide range of organic molecules.
- Natural Product Synthesis: The Aldol Condensation is frequently employed in the synthesis of complex natural products. Many natural products contain aldol subunits, and the ability to selectively form carbon-carbon bonds through the aldol reaction is crucial for the efficient synthesis of these compounds.
- Pharmaceutical Synthesis: β-Hydroxy carbonyl compounds obtained through the Aldol Condensation are important intermediates in the synthesis of pharmaceuticals. They can be further modified to introduce specific functional groups or stereochemistry required for biological activity.
- Flavor and Fragrance Synthesis: The Aldol Condensation is used in the synthesis of flavor and fragrance compounds. β-Hydroxy carbonyl compounds obtained through this reaction can serve as precursors for the synthesis of various aroma compounds found in perfumes, cosmetics, and food flavorings.
- Polyketide Synthesis: Polyketides are a class of natural products with diverse biological activities, including antibiotic, antifungal, and anticancer properties. The Aldol Condensation plays a crucial role in the biosynthesis of polyketides and is also utilized in the laboratory synthesis of polyketide analogs for medicinal purposes.
- Materials Science: β-Hydroxy carbonyl compounds synthesized via the Aldol Condensation can be used as building blocks in the preparation of functional materials, including polymers and surfactants.
- Asymmetric Synthesis: Enantioselective versions of the Aldol Condensation have been developed, enabling the synthesis of chiral β-hydroxy carbonyl compounds with high stereocontrol. These compounds are valuable in medicinal chemistry and asymmetric synthesis.
The Aldol Condensation is widely used in organic synthesis for the formation of complex molecules, including natural products, pharmaceuticals, and fine chemicals. It provides a versatile method for the construction of carbon-carbon bonds and the introduction of functional groups into organic molecules.















