The Barton-McCombie deoxygenation, also known as the Barton-McCombie reaction, is a chemical reaction used in organic synthesis to selectively remove oxygen functional groups, such as alcohols or ethers, from organic molecules. The reaction was developed by Derek H. R. Barton and Stuart McCombie in the 1970s.
The general reaction scheme for the Barton-McCombie deoxygenation is as follows:
R-O-R’+R”-H =[THF, refluxTi(III)Cl] R-R’+HOR”
Where:
- R, R’, and R” represent organic substituents.
- Ti(III)Cl refers to titanium(III) chloride, which acts as a reducing agent.
- THF stands for tetrahydrofuran, a common solvent used in the reaction.
The mechanism of the Barton-McCombie deoxygenation involves the generation of a titanium(III) species from titanium(IV) chloride and a reducing agent, typically an alkyl or aryl hydride. The titanium(III) species then reacts with the oxygen functional group, abstracting an oxygen atom to form a titanium oxo species. Subsequent reductive elimination releases the deoxygenated product and regenerates the titanium(III) species.
Key features and considerations of the Barton-McCombie deoxygenation include:
- Selectivity: The reaction is selective for the removal of oxygen functional groups, such as alcohols or ethers, while leaving other functional groups intact.
- Mild Conditions: The Barton-McCombie deoxygenation is typically conducted under relatively mild conditions, such as refluxing tetrahydrofuran (THF), making it compatible with a wide range of functional groups.
- Generality: The reaction is applicable to a variety of substrates containing oxygen functional groups, including complex molecules and natural products.
- Synthetic Utility: The deoxygenated products obtained from the Barton-McCombie reaction are valuable intermediates in organic synthesis, as they can undergo further functionalization or be used in the synthesis of more complex molecules.
Overall, the Barton-McCombie deoxygenation is a useful synthetic method for the selective removal of oxygen functional groups from organic molecules, providing access to a variety of deoxygenated products for use in organic synthesis and chemical transformations.
The Barton-McCombie deoxygenation mechanism involves the generation of a titanium(III) species, followed by its reaction with the oxygen-containing functional group in the substrate to remove the oxygen atom. Here’s a simplified outline of the mechanism:
- Generation of Titanium(III) Species: Titanium(III) species are generated in situ from titanium(IV) chloride (TiCl4) and a reducing agent, typically an alkyl or aryl hydride. The reducing agent donates a hydride ion (H−H−) to titanium(IV) chloride, leading to the formation of titanium(III) chloride (TiCl3TiCl3) and regenerating the reducing agent in the process.
TiCl4+H−→TiCl3+HCl
- Formation of Titanium Oxo Intermediate: The generated titanium(III) species reacts with the oxygen-containing functional group in the substrate. For example, in the case of an alcohol, the titanium(III) species abstracts an oxygen atom from the hydroxyl group to form a titanium oxo intermediate.
TiCl3+ROH→Ti(OR)Cl2+HCl
- Reductive Elimination: The titanium oxo intermediate undergoes reductive elimination, resulting in the cleavage of the carbon-oxygen bond. This process releases the deoxygenated product and regenerates the titanium(III) species, which can participate in further deoxygenation reactions.
Ti(OR)Cl2→R+TiCl3+ROCl
The overall transformation can be summarized as the removal of the oxygen atom from the substrate, resulting in the formation of a deoxygenated product (R) and an oxygen-containing byproduct (ROCl). The titanium(III) chloride catalyst remains in the reaction cycle, allowing for the successive deoxygenation of additional substrate molecules.
It’s important to note that while this mechanism provides a general overview of the Barton-McCombie deoxygenation, the exact details may vary depending on the specific substrate and reaction conditions. Additionally, the mechanism may involve the formation of various intermediates and transition states, which can influence the reaction kinetics and selectivity.
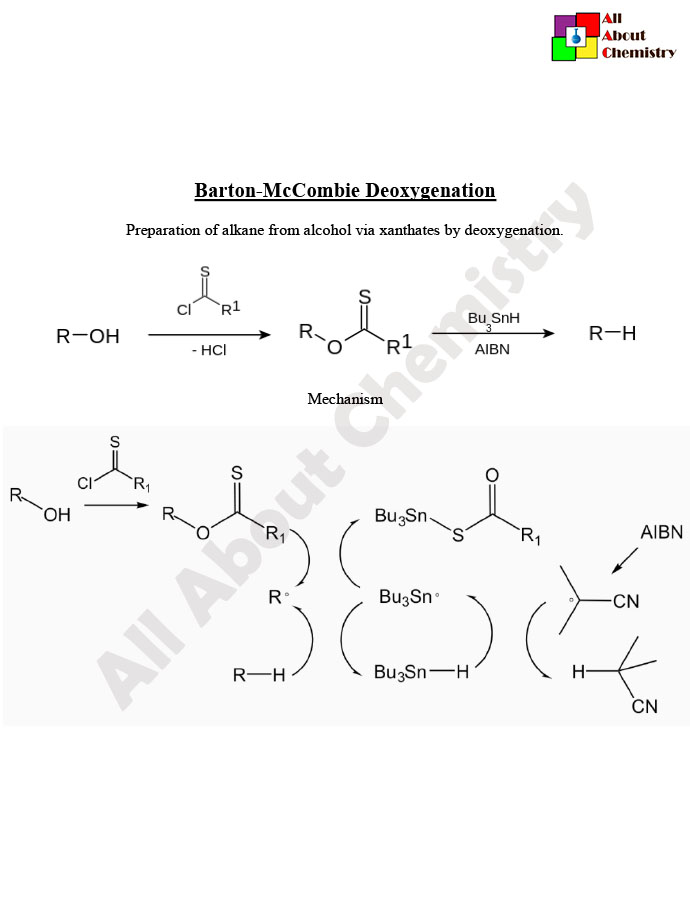
The Barton-McCombie deoxygenation reaction has found numerous applications in organic synthesis due to its ability to selectively remove oxygen functional groups from organic molecules. Some of the key applications include:
- Synthesis of Hydrocarbons: One of the primary applications of the Barton-McCombie deoxygenation is the synthesis of hydrocarbons from oxygen-containing compounds. By removing oxygen functional groups such as alcohols or ethers, this reaction enables the conversion of oxygenated organic molecules into hydrocarbons, which are valuable intermediates in the synthesis of various organic compounds.
- Natural Product Synthesis: The Barton-McCombie deoxygenation is widely used in the synthesis of natural products and pharmaceutical compounds. Many natural products contain oxygen functional groups that need to be removed or modified to access the desired target molecules. The selective deoxygenation provided by this reaction allows for the efficient synthesis of complex natural products and their analogs.
- Functional Group Interconversion: The deoxygenation reaction can be employed to interconvert different functional groups in organic molecules. For example, alcohols can be converted into corresponding alkyl groups, which can then undergo further functionalization reactions. This versatility makes the Barton-McCombie deoxygenation a valuable tool for synthetic chemists in designing synthetic routes towards target molecules.
- Polymer Chemistry: In polymer chemistry, the Barton-McCombie deoxygenation can be used for the modification of polymer structures. Oxygen-containing functional groups in polymers, such as hydroxyl groups, can be selectively removed to alter the properties of the polymer, such as solubility, thermal stability, or compatibility with other materials.
- Pharmaceuticals and Drug Discovery: The deoxygenation reaction is utilized in pharmaceutical research and drug discovery programs. Oxygen functional groups in drug candidates often need to be removed or modified to improve their pharmacological properties, such as bioavailability, metabolic stability, or binding affinity to target receptors. The Barton-McCombie deoxygenation provides a practical method for achieving such modifications in the molecular structure of drug candidates.
Overall, the Barton-McCombie deoxygenation reaction offers a versatile and efficient method for selectively removing oxygen functional groups from organic molecules, enabling diverse applications in organic synthesis, natural product synthesis, polymer chemistry, and pharmaceutical research. Its broad utility makes it a valuable tool in the toolkit of synthetic chemists aiming to access complex molecular architectures for various applications.















