Absolutely! Let’s delve into the fascinating world of aldehydes, ketones, and carboxylic acids, exploring their structures, properties, reactions, and applications.
Aldehydes, Ketones, and Carboxylic Acids: A Comprehensive Overview
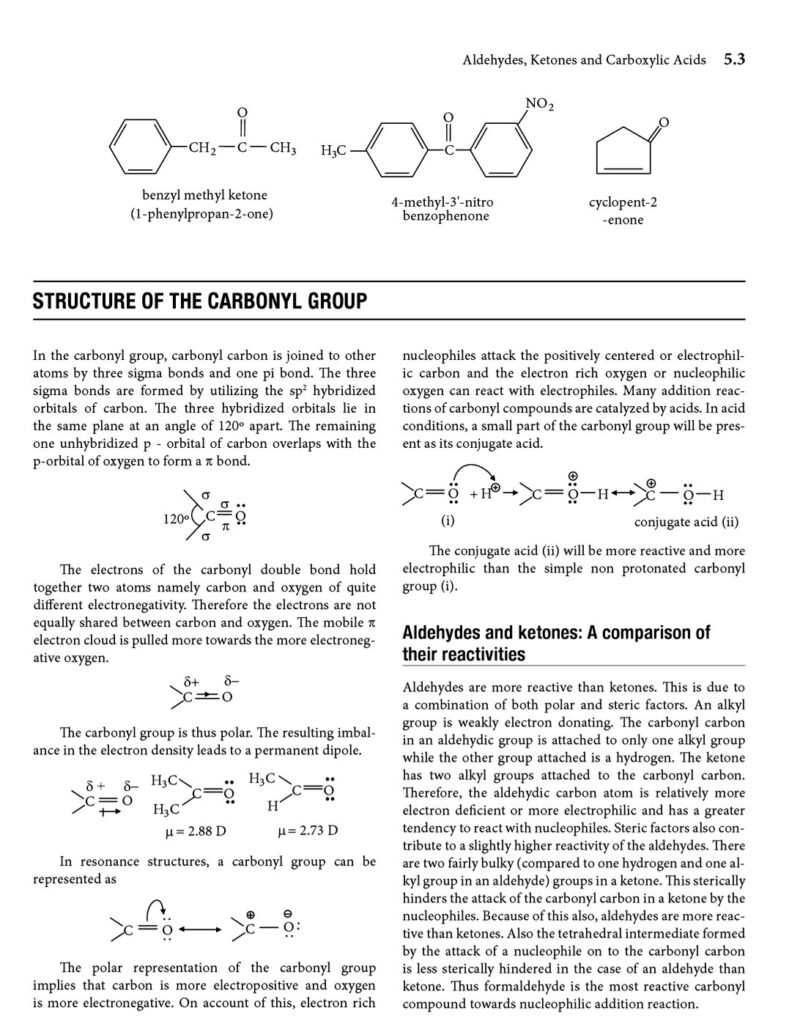
These three classes of organic compounds are characterized by the presence of the carbonyl group (C=O), a functional group that significantly influences their chemical behavior. While they share this common feature, subtle differences in their structures lead to distinct properties and reactivities.
1. Aldehydes:
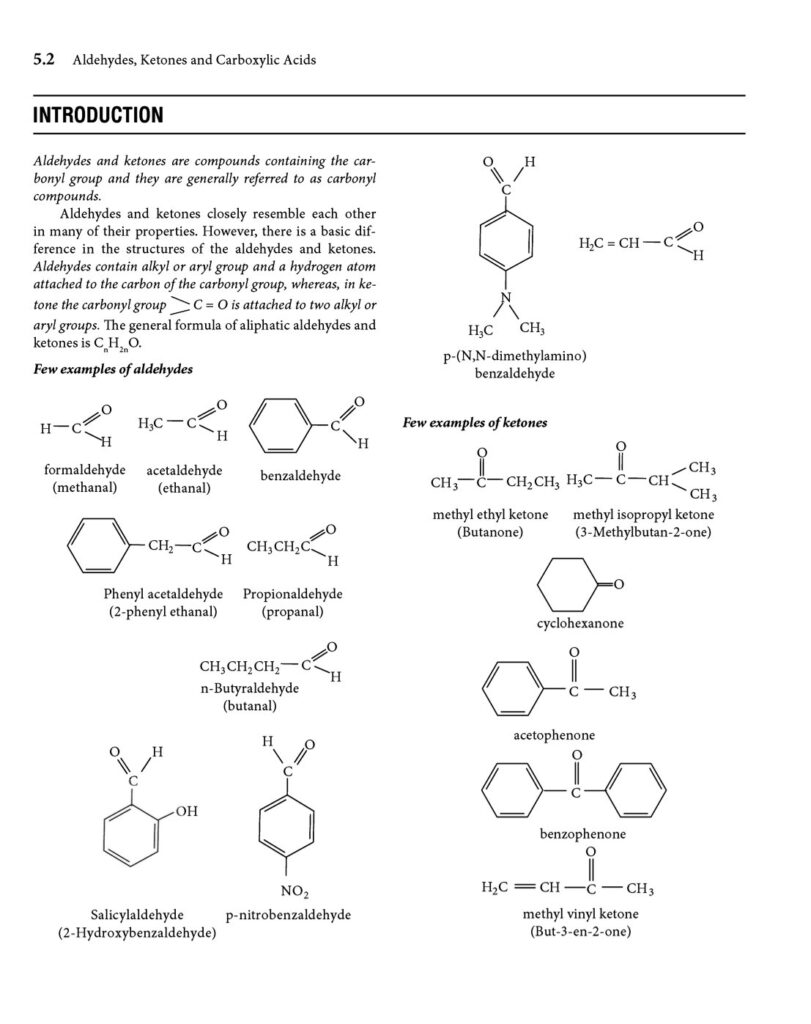
- Structure: Aldehydes possess a carbonyl group (C=O) where the carbon atom is bonded to at least one hydrogen atom and one alkyl or aryl group. The general formula is RCHO, where R represents an alkyl or aryl group, and CHO is the aldehyde functional group.
- Nomenclature:
- IUPAC: The suffix “-al” is added to the parent alkane name. For example, methanal (formaldehyde), ethanal (acetaldehyde), propanal.
- Common names: Many aldehydes have common names derived from the corresponding carboxylic acids. For example, formaldehyde, acetaldehyde, benzaldehyde.
- Properties:
- Polar molecules due to the presence of the carbonyl group.
- Lower aldehydes are gases or volatile liquids with pungent odors.
- Higher aldehydes are liquids or solids.
- Boiling points are higher than corresponding alkanes but lower than alcohols due to the absence of hydrogen bonding between aldehyde molecules.
- Reactions:
- Oxidation: Aldehydes are easily oxidized to carboxylic acids. This property is used in tests like Tollens’ reagent and Fehling’s solution.
- Reduction: Aldehydes can be reduced to primary alcohols using reducing agents like NaBH4 or LiAlH4.
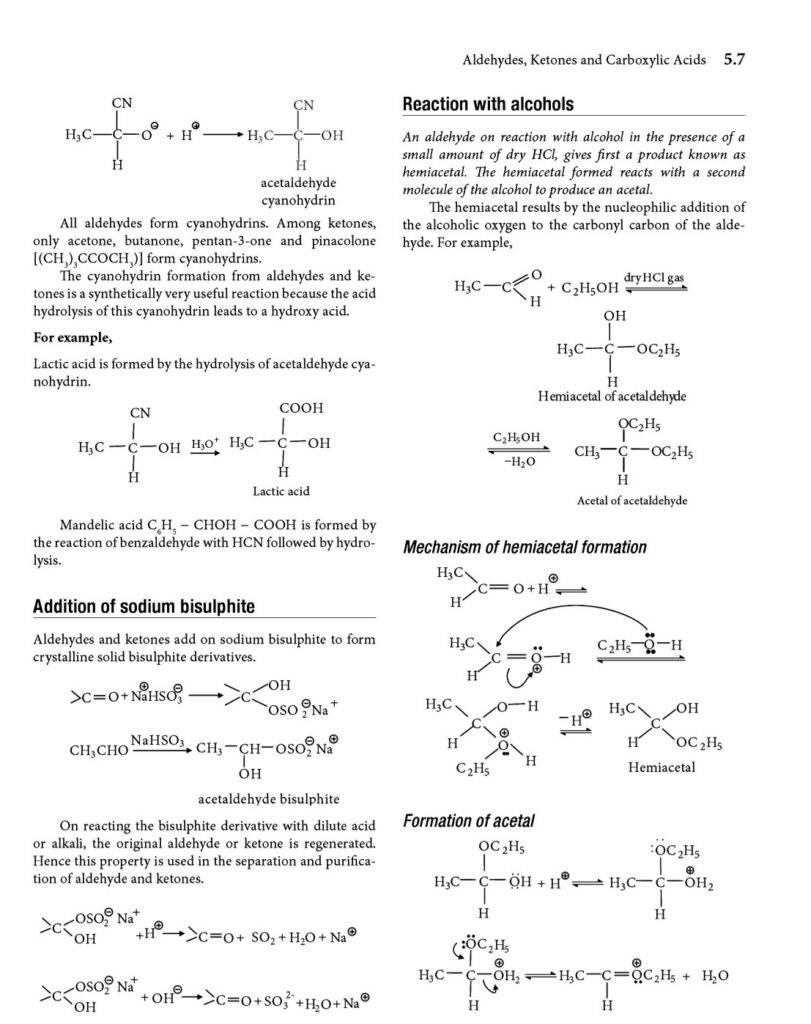
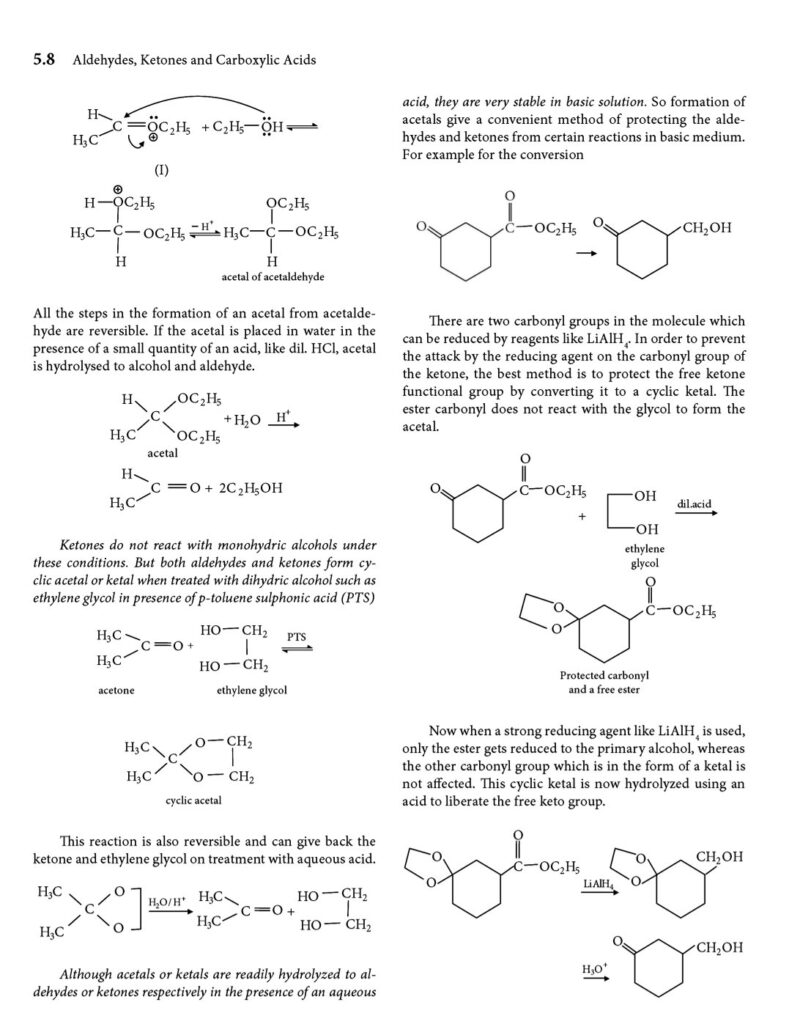
- Nucleophilic addition: the carbonyl carbon is electrophilic, and readily reacts with nucleophiles.
- Aldol condensation: Aldehydes with alpha-hydrogens can undergo aldol condensation in the presence of a base.
- Applications:
- Formaldehyde: Used in the production of resins, polymers, and disinfectants.
- Acetaldehyde: Used in the synthesis of acetic acid and other organic compounds.
- Benzaldehyde: used in flavorings.
2. Ketones:
- Structure: Ketones have a carbonyl group (C=O) where the carbon atom is bonded to two alkyl or aryl groups. The general formula is RCOR′, where R and R′ represent alkyl or aryl groups.
- Nomenclature:
- IUPAC: The suffix “-one” is added to the parent alkane name, and a number is used to indicate the position of the carbonyl group. For example, propanone (acetone), butanone.
- Common names: Acetone is a very common name.
- Properties:
- Polar molecules.
- Liquids with characteristic odors.
- Boiling points are higher than corresponding alkanes but lower than alcohols.
- Less reactive than aldehydes due to steric hinderance.
- Reactions:
- Reduction: Ketones can be reduced to secondary alcohols using reducing agents like NaBH4 or LiAlH4.
- Nucleophilic addition: the carbonyl carbon is electrophilic, and readily reacts with nucleophiles.
- Aldol condensation: Ketones with alpha-hydrogens can undergo aldol condensation in the presence of a base.
- Applications:
- Acetone: Used as a solvent, in nail polish remover, and in the production of various chemicals.
- Methyl ethyl ketone: solvent.
3. Carboxylic Acids:
- Structure: Carboxylic acids contain a carboxyl group (COOH), which consists of a carbonyl group (C=O) bonded to a hydroxyl group (OH). The general formula is RCOOH, where R represents an alkyl or aryl group.
- Nomenclature:
- IUPAC: The suffix “-oic acid” is added to the parent alkane name. For example, methanoic acid (formic acid), ethanoic acid (acetic acid), propanoic acid.
- Common names: Formic acid, acetic acid, benzoic acid are common names.
- Properties:
- Polar molecules due to the presence of the carboxyl group.
- Form hydrogen bonds, leading to higher boiling points compared to aldehydes and ketones of similar molecular weights.
- Lower carboxylic acids are liquids with pungent odors.
- Higher carboxylic acids are solids.
- Weak acids in aqueous solutions.
- Reactions:
- Acid-base reactions: Carboxylic acids react with bases to form carboxylate salts.
- Esterification: Carboxylic acids react with alcohols in the presence of an acid catalyst to form esters.
- Reduction: Carboxylic acids can be reduced to primary alcohols using strong reducing agents like LiAlH4.
- Decarboxylation: some carboxylic acids lose CO2 when heated.
- Applications:
- Acetic acid: Used in vinegar, food preservation, and the production of various chemicals.
- Formic acid: Used in the textile and leather industries.
- Benzoic acid: preservative.
- Fatty acids: components of lipids.
Key Differences and Relationships:
- Oxidation States: Aldehydes are intermediate oxidation products between primary alcohols and carboxylic acids. Ketones are intermediate oxidation products between secondary alcohols and tertiary alcohols, which cannot be oxidized without breaking carbon carbon bonds.
- Reactivity: Aldehydes are generally more reactive than ketones due to less steric hindrance and the presence of a hydrogen atom on the carbonyl carbon. Carboxylic acids, while reactive, exhibit different reactivity patterns due to the presence of the hydroxyl group.
- Hydrogen bonding: carboxylic acids form very strong hydrogen bonds because of the two oxygen atoms, making their boiling points relatively high.
Summary Table:
| Feature | Aldehydes (RCHO) | Ketones (RCOR′) | Carboxylic Acids (RCOOH) |
| Functional Group | CHO | C=O | COOH |
| Nomenclature | -al | -one | -oic acid |
| Oxidation | Easily oxidized | Difficult to oxidize | Resistant to oxidation |
| Reduction | Primary alcohols | Secondary alcohols | Primary alcohols |
| Acidity | Neutral | Neutral | Acidic |
| Common uses | Formaldehyde, Acetaldehyde, Benzaldehyde | Acetone, MEK | Acetic acid, fatty acids |
This comprehensive overview provides a foundation for understanding the chemistry of aldehydes, ketones, and carboxylic acids. Their diverse properties and reactivities make them essential compounds in organic chemistry and various industrial applications.