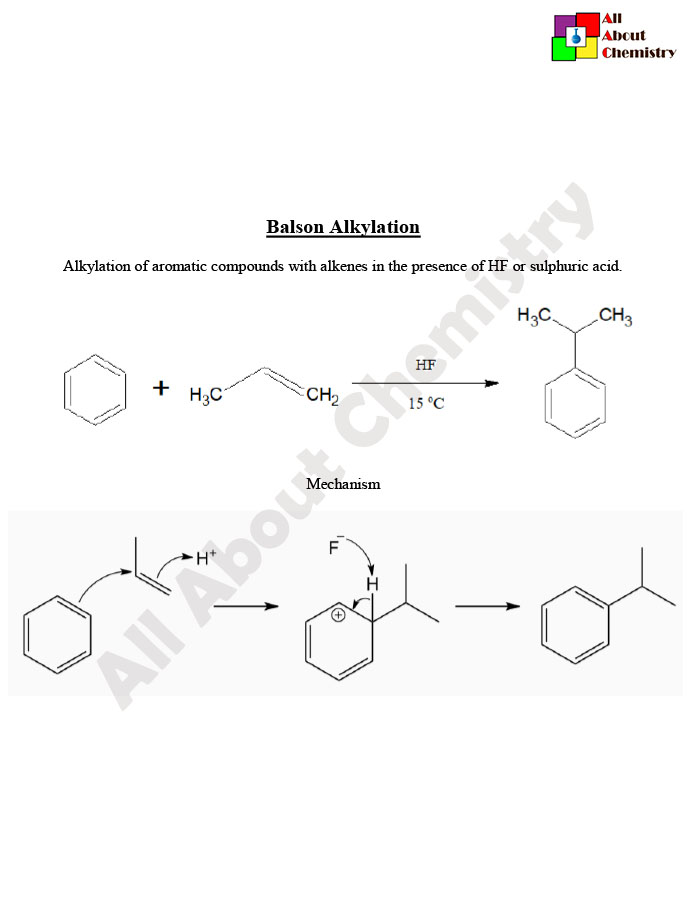
Balson alkylation is a method used in organic synthesis for the selective alkylation of phenols or aromatic amines. The process involves the use of aluminum chloride (AlCl3) as a Lewis acid catalyst along with alkyl halides, typically alkyl chlorides or alkyl bromides, as the alkylating agents.
The general reaction scheme for the Balson alkylation is as follows:
Phenol (or aromatic amine)+Alkyl halide→Alkylated phenol (or aromatic amine)+HCl (or HBr)
The mechanism involves the formation of a complex between the Lewis acid catalyst (AlCl3) and the alkyl halide, facilitating the electrophilic substitution reaction between the aromatic compound (phenol or aromatic amine) and the alkyl group. The Lewis acid activates the alkyl halide, making it more susceptible to attack by the nucleophilic aromatic compound.
Key features and considerations of the Balson alkylation include:
- Selective Alkylation: Balson alkylation allows for the selective alkylation of phenols or aromatic amines in the presence of other functional groups. The Lewis acid catalyst promotes the desired alkylation reaction while minimizing side reactions.
- Scope: This method is applicable to a wide range of phenols and aromatic amines, providing access to various alkylated derivatives.
- Regioselectivity: The regioselectivity of the alkylation reaction depends on factors such as the nature of the aromatic compound and the substitution pattern of the alkyl halide. However, in many cases, the reaction proceeds with good regioselectivity.
- Lewis Acid Catalysis: The use of aluminum chloride as a Lewis acid catalyst is essential for promoting the reaction between the aromatic compound and the alkyl halide. Other Lewis acids, such as iron(III) chloride (FeCl3), can also be employed in similar processes.
- Precautions: Balson alkylation reactions often require careful control of reaction conditions, including temperature, stoichiometry, and choice of solvent, to optimize yields and selectivity. Additionally, handling of aluminum chloride and alkyl halides should be conducted with appropriate safety precautions due to their reactivity and potential hazards.
Overall, Balson alkylation provides a valuable synthetic route for the introduction of alkyl groups onto aromatic compounds, offering versatility and selectivity in the functionalization of phenols and aromatic amines in organic synthesis.
Balson alkylation, also known as Friedel-Crafts alkylation, follows a mechanism that involves the activation of the alkyl halide by a Lewis acid catalyst, typically aluminum chloride (AlCl3), followed by electrophilic aromatic substitution with the aromatic compound (phenol or aromatic amine). Here’s a general outline of the mechanism:
- Formation of the Acylium Ion: The Lewis acid catalyst, often aluminum chloride (AlCl3), activates the alkyl halide by forming a complex with it. This complexation generates an acylium ion (or alkyl cation), which is the electrophile in the subsequent reaction.
AlCl3+R-X→AlCl3-R++X−
- Electrophilic Attack: The activated alkyl cation acts as an electrophile and reacts with the aromatic compound (phenol or aromatic amine). In the case of phenol, the nucleophilic aromatic ring attacks the electrophilic carbocation, leading to the substitution of a hydrogen atom with the alkyl group.
Ar-H+AlCl3-R+→Ar-R+AlCl3+H+
- Regeneration of the Catalyst: After the electrophilic substitution, the Lewis acid catalyst is regenerated, ready to participate in another cycle of the reaction. The resulting alkylated aromatic compound is now the desired product.
AlCl3+HCl
It’s important to note that Balson alkylation is subject to issues such as polyalkylation and rearrangement, which can lead to the formation of undesired byproducts. Therefore, reaction conditions must be carefully controlled to minimize these side reactions and achieve high selectivity and yield of the desired alkylated product. Additionally, other variations of Friedel-Crafts alkylation, such as the Schollkopf-Barton-Zard (SBZ) reaction, have been developed to address some of these challenges.
Balson alkylation, also known as Friedel-Crafts alkylation, has several important applications in organic synthesis:
- Introduction of Alkyl Groups: Balson alkylation is widely used for the introduction of alkyl groups onto aromatic compounds such as benzene derivatives, phenols, and aromatic amines. This method allows chemists to modify the chemical properties of aromatic compounds by incorporating alkyl substituents, thereby enhancing their reactivity, solubility, or biological activity.
- Synthesis of Pharmaceuticals: Balson alkylation plays a key role in the synthesis of pharmaceutical compounds. By selectively alkylating aromatic rings in drug molecules, chemists can modulate the pharmacological properties of these compounds, such as their potency, selectivity, and metabolic stability. Many drugs, including analgesics, antihistamines, and antipsychotics, contain alkylated aromatic moieties synthesized via Balson alkylation.
- Preparation of Fragrances and Flavor Compounds: Balson alkylation is employed in the synthesis of fragrances and flavor compounds used in perfumery, cosmetics, and food industries. By introducing alkyl groups onto aromatic rings, chemists can create new aromatic compounds with unique olfactory and gustatory properties. This method allows for the production of a wide range of synthetic fragrances and flavor additives that mimic natural aromas and tastes.
- Materials Chemistry: Balson alkylation is utilized in materials chemistry for the functionalization of aromatic polymers and resins. By incorporating alkyl substituents into the polymer backbone or side chains, chemists can modify the physical and chemical properties of polymers, such as their mechanical strength, thermal stability, and solubility. This method enables the synthesis of customized polymer materials tailored for specific applications in coatings, adhesives, and engineering plastics.
- Agrochemical Synthesis: Balson alkylation is employed in the synthesis of agrochemicals such as pesticides, herbicides, and fungicides. By alkylating aromatic rings in pesticide molecules, chemists can enhance their efficacy, stability, and environmental compatibility. This method allows for the development of novel agrochemicals with improved pest control properties and reduced environmental impact.
- Natural Product Synthesis: Balson alkylation is utilized in the synthesis of natural products and their derivatives. Many naturally occurring compounds, such as alkaloids, flavonoids, and lignans, contain alkylated aromatic moieties synthesized via Balson alkylation. By mimicking these structural motifs, chemists can access diverse natural product analogs for biological evaluation and drug discovery efforts.
Overall, Balson alkylation is a versatile and widely used synthetic method for the functionalization of aromatic compounds, with applications spanning from pharmaceuticals and fragrances to materials science and agrochemistry. Its ability to introduce alkyl substituents selectively onto aromatic rings makes it a valuable tool for synthetic chemists seeking to access diverse molecular architectures for various applications.








