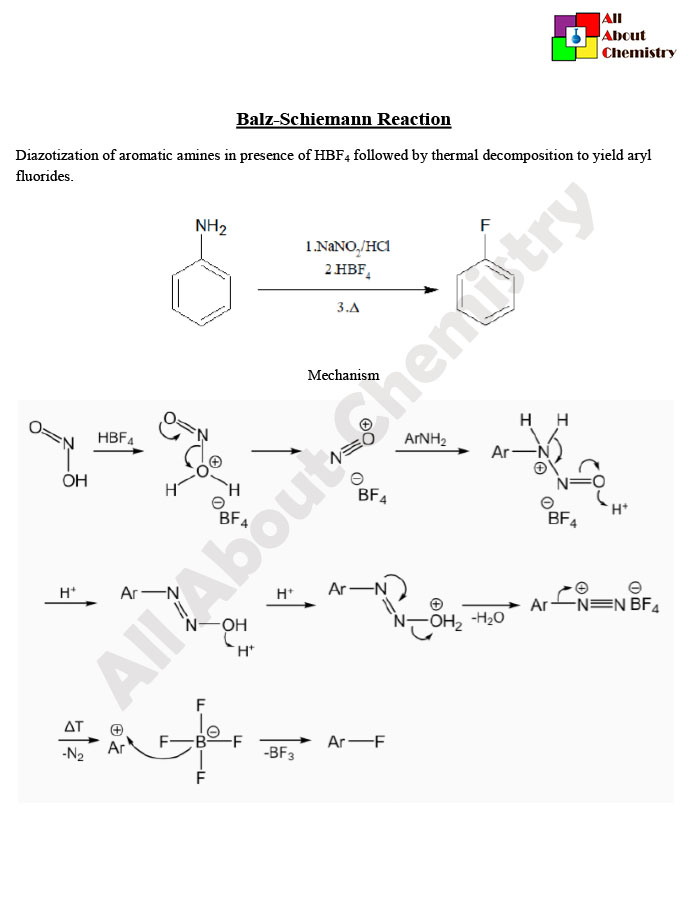
The Balz-Schiemann reaction, also known as aryl diazonium salt decomposition, is a chemical reaction used to replace a diazonium group (RN2+) with a halogen atom on an aromatic ring. It is named after the German chemists Balz and Schiemann, who first described the reaction in the early 20th century.
The general reaction scheme for the Balz-Schiemann reaction is as follows:
ArN2+X−+Halogen (X2)→ArX+N2+HX
Where:
- Ar represents an aryl group attached to the diazonium ion (RN2+).
- X represents a halogen atom, typically fluorine (F), chlorine (Cl), or bromine (Br).
- ArX represents the halogenated aromatic compound.
- N2 represents nitrogen gas.
- HX represents the hydrogen halide produced as a byproduct.
The Balz-Schiemann reaction proceeds via a diazonium salt intermediate, which is formed by the diazotization of an aromatic amine with nitrous acid (HNO2). This intermediate is then treated with a halogen source, such as chlorine gas (Cl2) or bromine (Br2), to replace the diazonium group with a halogen atom. The reaction typically occurs under acidic conditions.
The Balz-Schiemann reaction, also known as the diazonium salt decomposition reaction, involves the replacement of a diazonium group (RN2+) with a halogen atom on an aromatic ring. The mechanism of this reaction can vary depending on the specific conditions and halogen source used. Here’s a general outline of the mechanism:
- Formation of Diazonium Salt: The reaction begins with the formation of the diazonium salt intermediate by diazotization of an aromatic amine with nitrous acid (HNO2). This step involves the protonation of the amine followed by the conversion of the amino group to a diazonium ion.
ArNH2+HNO2→ArN2+X−+H2O+N2
- Electrophilic Attack by Halogen: In the presence of a halogen source, such as chlorine gas (Cl2) or bromine (Br2), the diazonium salt undergoes electrophilic aromatic substitution. The halogen atom acts as the electrophile and attacks the aromatic ring, displacing the diazonium group.
ArN2+X−+X2→ArX+N2+HX
- Rearrangement and Elimination: The intermediate formed after the electrophilic attack may undergo rearrangement or elimination processes, depending on the specific reaction conditions and the nature of the aromatic substrate. Rearrangement can occur to stabilize the intermediate, while elimination may result in the formation of byproducts.
- Formation of Halogenated Product: The final outcome of the reaction is the formation of the halogenated aromatic compound (ArX) as the main product, along with nitrogen gas (N2) and a hydrogen halide byproduct (HX).
Overall, the Balz-Schiemann reaction proceeds through a combination of diazotization, electrophilic aromatic substitution, and subsequent rearrangement or elimination steps. The reaction allows for the introduction of halogen substituents onto aromatic rings and finds utility in various organic synthesis applications, particularly in the preparation of halogenated aromatic compounds for further functionalization.
The Balz-Schiemann reaction, also known as diazonium salt decomposition, has several important applications in organic synthesis and materials science:
- Aromatic Halogenation: The Balz-Schiemann reaction is widely used for the direct halogenation of aromatic compounds. By converting diazonium salts into halogenated aromatic compounds, this reaction allows for the introduction of halogen substituents (such as fluorine, chlorine, or bromine) onto aromatic rings. These halogenated aromatic compounds are valuable intermediates in the synthesis of pharmaceuticals, agrochemicals, dyes, and other organic molecules.
- Synthesis of Aryl Halides: Balz-Schiemann reaction serves as a convenient method for the preparation of aryl halides, which are important building blocks in organic synthesis. Aryl halides find extensive use in cross-coupling reactions (e.g., Suzuki coupling, Heck reaction) to form carbon-carbon or carbon-heteroatom bonds. These reactions enable the construction of complex organic molecules, including natural products, pharmaceuticals, and materials.
- Functionalization of Aromatic Compounds: The Balz-Schiemann reaction allows for the selective functionalization of aromatic compounds by introducing halogen substituents at specific positions on the aromatic ring. This functionalization can be further elaborated through subsequent reactions to introduce other functional groups or modify the chemical properties of the aromatic molecule. The versatility of this reaction makes it a valuable tool in synthetic organic chemistry.
- Materials Science: In materials science, the Balz-Schiemann reaction is utilized for the modification of aromatic polymers and surfaces. By introducing halogen groups onto aromatic substrates, researchers can tailor the surface properties of materials, such as wettability, adhesion, and chemical reactivity. Halogenated aromatic compounds are also used as precursors for the synthesis of functionalized polymers, coatings, and materials with specific properties for applications in electronics, optics, and biotechnology.
- Dye Synthesis: Balz-Schiemann reaction is employed in the synthesis of azo dyes, which are extensively used in the textile, printing, and coloring industries. Azo dyes are synthesized by coupling diazonium salts with aromatic compounds containing suitable functional groups under alkaline conditions. The resulting azo compounds exhibit vivid colors and are widely used as colorants in various applications.
Overall, the Balz-Schiemann reaction offers a versatile and widely applicable method for the introduction of halogen substituents onto aromatic compounds. Its utility in organic synthesis, materials science, and dye chemistry makes it an important tool for the preparation of diverse organic molecules and functional materials.















