The Bouveault-Blanc reduction, also known simply as the Blanc reaction, is a chemical reaction named after French chemists Louis Bouveault and Gustave Louis Blanc. It’s a variation of the Bouveault synthesis and is used specifically for the reduction of esters to alcohols. This method is notable for its ability to selectively reduce esters to aldehydes, which can then be further reduced to primary alcohols, thus providing a method for the controlled synthesis of aldehydes and primary alcohols.
The general reaction scheme for the Bouveault-Blanc reduction is as follows:
R-COOR’+2Na+2ROH→R-CHO+R’OH+2NaOH
In this reaction, an ester (R-COOR’) reacts with sodium metal (NaNa) in the presence of an alcohol solvent (ROH), typically ethanol or methanol, to yield an aldehyde (R-CHOR-CHO) and an alcohol (R’OH), along with sodium hydroxide (NaOH) as a byproduct.
The Bouveault-Blanc reduction proceeds through a radical mechanism similar to the Bouveault synthesis, where the sodium metal generates radicals that initiate the reduction process. The reaction conditions are carefully controlled to ensure the selective formation of aldehydes, which can be further reduced if desired to obtain primary alcohols.
The Bouveault-Blanc reduction has found numerous applications in organic synthesis, particularly in the preparation of aldehydes and primary alcohols for use as intermediates in the synthesis of various organic compounds, including pharmaceuticals, fragrances, and fine chemicals. It offers a versatile and selective method for the reduction of esters, allowing chemists to access a wide range of functionalized aldehydes and primary alcohols with control over regioselectivity and stereochemistry.
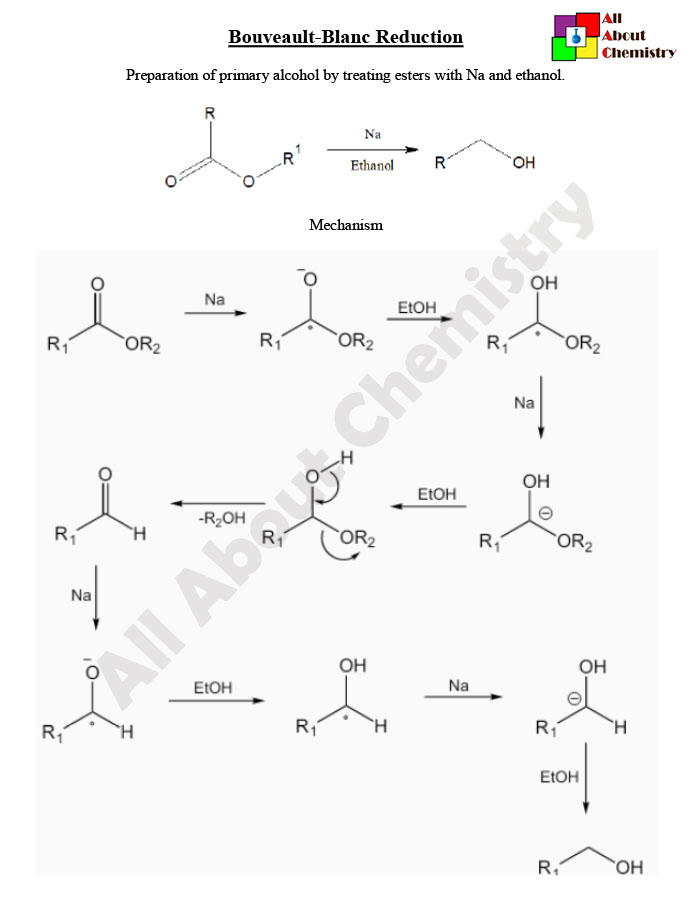
The mechanism of the Bouveault-Blanc reduction involves several steps and intermediates. Here’s a simplified overview of the mechanism:
- Formation of Sodium Alkoxide: Sodium metal (Na) reacts with the alcohol solvent (ROH) to form sodium alkoxide (NaOR) and hydrogen gas (H2). This step is often initiated by the presence of a trace amount of alcohol in the reaction mixture.
- Formation of Alkoxysodium Intermediate: The alkoxide ion (RO−) reacts with the ester (R-COOR’) to form an alkoxysodium intermediate (R-COONa).
- Radical Formation: The alkoxysodium intermediate undergoes a radical mechanism where a single electron transfer occurs between the alkoxysodium intermediate and a sodium atom. This leads to the formation of a radical species.
- Hydrogen Abstraction: The radical species (R-COO∙) abstracts a hydrogen atom from another molecule of alcohol (ROH), forming a new radical intermediate and a molecule of water (H2O).
- Aldehyde Formation: The radical intermediate (RO∙) reacts with another molecule of alkoxide (RO−) to form an aldehyde (R-CHOR-CHO) and regenerate the alkoxide.
- Formation of Primary Alcohol: The aldehyde (R-CHOR-CHO) formed in the previous step can further react with sodium metal and alcohol solvent to form a primary alcohol (R’OH).
Overall, the Bouveault-Blanc reduction proceeds through a series of radical-mediated steps, ultimately leading to the reduction of esters to aldehydes, which can then be further reduced to primary alcohols if desired. The reaction conditions, particularly the choice of alcohol solvent and reaction temperature, are crucial for controlling the selectivity and efficiency of the reaction.
The Bouveault-Blanc reduction, also known as the Blanc reaction, finds various applications in organic synthesis due to its ability to selectively reduce esters to aldehydes. Here are some of its key applications:
- Aldehyde Synthesis: One of the primary applications of the Bouveault-Blanc reduction is the synthesis of aldehydes from esters. The selective reduction of esters to aldehydes is valuable because aldehydes are versatile intermediates in organic synthesis. They can undergo various transformations, such as nucleophilic addition reactions, to yield a wide range of functionalized compounds.
- Carbonyl Group Manipulation: The Bouveault-Blanc reduction allows for the manipulation of carbonyl groups in organic molecules. By selectively converting esters to aldehydes, chemists can introduce aldehyde functionalities into complex molecules, enabling further synthetic elaboration.
- Natural Product Synthesis: The synthesis of natural products often requires the introduction of aldehyde functionalities at specific positions within the molecule. The Bouveault-Blanc reduction provides a useful method for achieving this, allowing chemists to access key intermediates for the synthesis of complex natural products.
- Pharmaceutical Synthesis: Aldehydes are important intermediates in the synthesis of pharmaceutical compounds. The Bouveault-Blanc reduction can be employed in the synthesis of pharmaceutical intermediates or key building blocks, enabling the efficient preparation of bioactive molecules and drug candidates.
- Flavor and Fragrance Synthesis: Many flavor and fragrance compounds contain aldehyde functionalities, which contribute to their characteristic odor and taste. The Bouveault-Blanc reduction can be used in the synthesis of aldehyde-containing fragrance and flavor molecules, allowing for the preparation of natural and synthetic aromas and flavors.
- Functional Group Interconversion: The Bouveault-Blanc reduction provides a method for interconverting functional groups in organic molecules. By converting esters to aldehydes, chemists can alter the chemical properties of a compound, enabling further synthetic manipulations or facilitating the isolation of specific intermediates.
- Stereochemical Control: In certain cases, the Bouveault-Blanc reduction can provide stereochemical control over the formation of aldehydes. By carefully selecting reaction conditions, including the choice of solvent and temperature, chemists can influence the stereochemistry of the reduction process, leading to the formation of stereoselective aldehyde products.
Overall, the Bouveault-Blanc reduction is a valuable tool in organic synthesis, offering chemists a selective and versatile method for the synthesis of aldehydes and the manipulation of carbonyl groups in complex molecules. Its applications span various fields, including pharmaceuticals, natural product synthesis, flavor and fragrance chemistry, and fine chemicals synthesis.















