Collman carbonylation refers to a chemical reaction discovered by Henry Taube and Richard R. Squires in 1964. It involves the catalytic conversion of carbon monoxide (CO) and a metal carbonyl complex into an organic carbonyl compound. This reaction is significant in organometallic chemistry and has found applications in the synthesis of various organic compounds, including pharmaceuticals and fine chemicals.
The reaction typically proceeds with a metal carbonyl complex, often a transition metal such as nickel or iron, which reacts with CO to form a metal acyl complex. The metal acyl complex then reacts with a nucleophile, such as an alkene or an alcohol, to yield the desired organic carbonyl compound. The overall reaction can be summarized as follows:
Metal Carbonyl Complex + CO → Metal Acyl Complex
Metal Acyl Complex + Nucleophile → Organic Carbonyl Compound + Metal Complex
Collman carbonylation has been extensively studied and optimized over the years to improve its efficiency and selectivity. It has become a valuable tool in organic synthesis, particularly in the preparation of aldehydes, ketones, and carboxylic acids. Additionally, the reaction mechanism and factors affecting its outcome have been thoroughly investigated, contributing to a better understanding of organometallic chemistry.
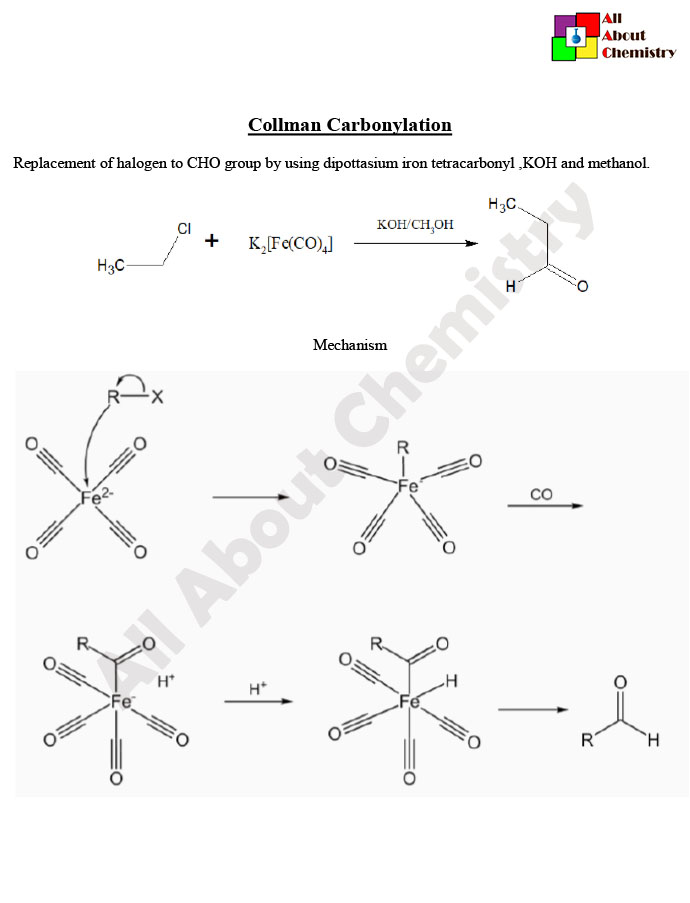
The mechanism of Collman carbonylation involves several steps, and it can vary depending on the specific metal carbonyl complex and reaction conditions. However, a general mechanism for Collman carbonylation typically involves the following steps:
- Activation of Metal Carbonyl Complex: Initially, the metal carbonyl complex undergoes activation by dissociating one of the carbonyl ligands. This step typically involves coordination of the CO ligand to a vacant site on the metal center.
- Addition of Carbon Monoxide (CO): The activated metal center then reacts with carbon monoxide (CO), which coordinates to the metal center to form a metal carbonyl intermediate.
- Migration of Carbonyl Ligand: In some cases, there may be a migration of the carbonyl ligand from the metal center to a coordinated ligand on the metal complex, leading to the formation of a metal acyl complex.
- Nucleophilic Attack: The metal acyl complex reacts with a nucleophile, which can be an alkene, an alcohol, or another suitable substrate. The nucleophile attacks the metal-bound carbonyl carbon, leading to the formation of a new carbon-carbon or carbon-oxygen bond, depending on the nature of the nucleophile.
- Rearrangement and Product Formation: Depending on the specific reaction conditions and the nature of the nucleophile, further rearrangement steps may occur, leading to the formation of the final organic carbonyl compound.
Overall, Collman carbonylation involves the activation of a metal carbonyl complex, coordination of carbon monoxide, nucleophilic attack, and subsequent rearrangement steps to yield the desired organic carbonyl compound. The exact mechanism may vary depending on factors such as the metal complex, ligands, and reaction conditions.
Collman carbonylation has several important applications in organic synthesis, particularly in the preparation of various organic compounds. Some of the key applications include:
- Synthesis of Aldehydes and Ketones: Collman carbonylation is commonly used in the synthesis of aldehydes and ketones. By reacting metal carbonyl complexes with carbon monoxide and suitable nucleophiles such as alkenes or alcohols, a wide range of aldehydes and ketones can be prepared efficiently.
- Preparation of Carboxylic Acids: Collman carbonylation can also be employed for the synthesis of carboxylic acids. By utilizing appropriate nucleophiles or by further oxidation of the intermediate aldehyde or ketone products, carboxylic acids can be obtained.
- Functional Group Interconversion: Collman carbonylation reactions can facilitate the interconversion of functional groups. For example, it can convert alkynes to enones, alkenes to ketones or aldehydes, and alcohols to carbonyl compounds.
- Total Synthesis of Natural Products: Collman carbonylation reactions have been utilized in the total synthesis of various natural products and pharmaceutical compounds. The versatility of this reaction allows chemists to introduce carbonyl groups selectively and efficiently, enabling the construction of complex molecular architectures.
- Fine Chemicals and Pharmaceutical Synthesis: The ability to selectively introduce carbonyl groups makes Collman carbonylation an important tool in the synthesis of fine chemicals and pharmaceutical intermediates. It offers a route to various key building blocks and functional groups commonly found in drug molecules.
- Catalytic Processes: Recent developments in Collman carbonylation have focused on catalytic processes, where transition metal catalysts facilitate the reaction under milder conditions, improving efficiency and selectivity while minimizing waste generation.
Overall, Collman carbonylation is a versatile and widely used synthetic tool with applications ranging from the preparation of simple carbonyl compounds to the synthesis of complex natural products and pharmaceuticals. Its importance lies in its ability to introduce carbonyl functionality selectively and efficiently, making it invaluable in modern organic synthesis.















