Welcome to an in-depth study of acids, bases, and salts, the very building block of ICSE Chemistry! The article is aimed at de-mystifying these important chemical concepts and providing you with the information and insight necessary to succeed in your studies and approach competency-based questions (CFQs) with confidence.
Acids, bases, and salts are not mere theoretical concepts limited to textbooks; they are part of our everyday life, from the food we eat to the medicines we consume. It is essential to know their properties, reactions, and uses in order to develop a strong foundation in chemistry.
If you’re having trouble understanding the pH concept, interested in the acid-base reaction, or looking for practical advice on how to tackle CFQs, you’ve come to the right place. We will demystify difficult concepts, give you real-life examples, and include practice questions to cement your understanding. So let’s set out on this thrilling adventure into acids, bases, and salts!
SECTION A
- Assertion (A): Acids are compounds that furnish H₃O⁺ ions in aqueous solutions.
Reason (R): Acids react with bases to form salt and water.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion (A): Strong acids undergo almost complete ionization in aqueous solutions.
Reason (R): The degree of ionization of a strong acid is less than 30%.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion (A): HCl is a monobasic acid.
Reason (R): HCl donates one H⁺ ion per molecule in aqueous solution.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion (A): Aqueous solutions of strong bases have a high pH value.
Reason (R): Strong bases undergo complete ionization and release a large number of OH⁻ ions.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion (A): A weak acid has a lower degree of ionization than a strong acid.
Reason (R): The degree of ionization of a weak acid is less than 30%.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion (A): NH₄OH is a weak alkali.
Reason (R): NH₄OH undergoes partial ionization in aqueous solution.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion (A): A solution of Na₂CO₃ is basic in nature.
Reason (R): Carbonate ions (CO₃²⁻) react with water to form OH⁻ ions.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion (A): Citric acid is a tribasic acid.
Reason (R): Citric acid furnishes three hydronium (H₃O⁺) ions per molecule in an aqueous solution.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion (A): The reaction of an acid with a metal carbonate produces carbon dioxide gas.
Reason (R): Carbon dioxide gas turns lime water milky.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion (A): pH of a strong acid solution is always lower than 7.
Reason (R): Strong acids have a higher concentration of H⁺ ions, making the solution highly acidic.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion: Strong acids have a high degree of ionization in aqueous solution.
Reason: Strong acids produce a large amount of hydronium ions (H3O+) when dissolved in water.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion: Sodium hydroxide (NaOH) is considered a strong alkali.
Reason: NaOH completely dissociates in water to produce a large number of hydroxide ions (OH-).
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion: When an acid reacts with a carbonate, hydrogen gas is evolved.
Reason: The reaction between an acid and a carbonate produces carbon dioxide, water, and a salt.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion: Phenolphthalein turns pink in an acidic solution.
Reason: Phenolphthalein is an acid-base indicator that changes colour based on the pH of the solution, turning pink in basic solutions.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion: The pH of a neutral solution at 25°C is 7.
Reason: pH is defined as the negative logarithm (base 10) of the hydrogen ion concentration, and in a neutral solution, [H+] = [OH-] = 10-7 M.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion: Salts formed from a strong acid and a weak base are acidic.
Reason: The anion from the strong acid hydrolyzes water, producing excess H+ ions.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion: Methyl orange turns yellow in a basic solution.
Reason: Methyl orange is an indicator that changes colour depending on the concentration of OH- ions.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion: Antacids are used to relieve acidity in the stomach.
Reason: Antacids neutralize excess hydrochloric acid (HCl) present in the stomach.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion: The basicity of an acid is the number of replaceable hydrogen atoms per molecule.
Reason: The basicity determines the number of hydronium ions (H3O+) an acid can furnish in an aqueous solution.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
- Assertion: All metal oxides are basic in nature.
Reason: Metal oxides react with water to form metal hydroxides, which are bases.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
SECTION B
- The degree of ionization of a weak acid is typically _______________________________________ 30%, indicating that it undergoes _______________________________________ dissociation in water. (below / above, partial / complete)
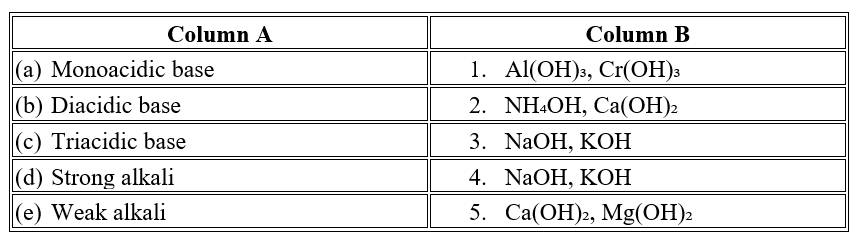
- A base that furnishes two hydroxide ions per molecule is called a ___________ base, and an example from the text is ___________. (monoacidic / diacidic, NaOH / Ca(OH)2)
- Acids formed by dissolving acidic oxides in water, such as H2SO4, are produced when ___________ reacts with water. (SO2 / SO3, SO3 / CO2)
- When a metal like zinc reacts with sulfuric acid, the gas evolved, ___________, burns with a ___________ sound. (CO2 / H2, pop / fizz)
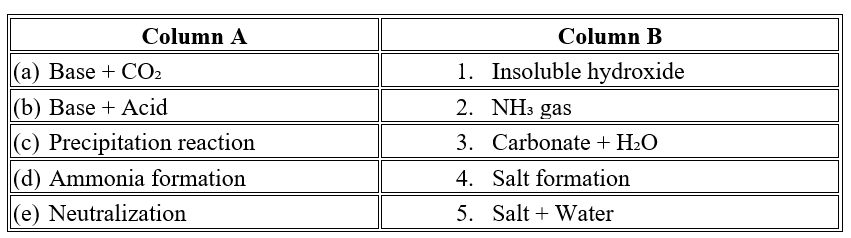
- The reaction between a base like NaOH and carbon dioxide produces ___________ and water, demonstrating the ___________ nature of metal oxides. (Na2CO3 / NaCl, acidic / basic)
- The pH of a solution ___________ with an increase in temperature, implying that the concentration of H+ ions ___________. (increases / decreases, increases / decreases)
- Salts formed from a strong acid and a weak base result in a solution that is ___________, due to the ___________ of the anion from the strong acid. (acidic / basic, hydrolysis / precipitation)
- An indicator like methyl orange turns ___________ in a basic solution, indicating a pH ___________ 7. (red / yellow, below / above)
- The formation of ammonia gas when heating an ammonium salt with a base like NaOH demonstrates the presence of ___________ ions and the ___________ nature of ammonia. (NH4+ / OH-, acidic / basic)
- The preparation of a metal oxide like ZnO by heating its carbonate salt, ZnCO3, involves a ___________ reaction, releasing ___________ gas. (decomposition / synthesis, CO2 / O2)
- Acids are compounds that furnish _______________________________________ ions in aqueous solution.( H+ / OH- )
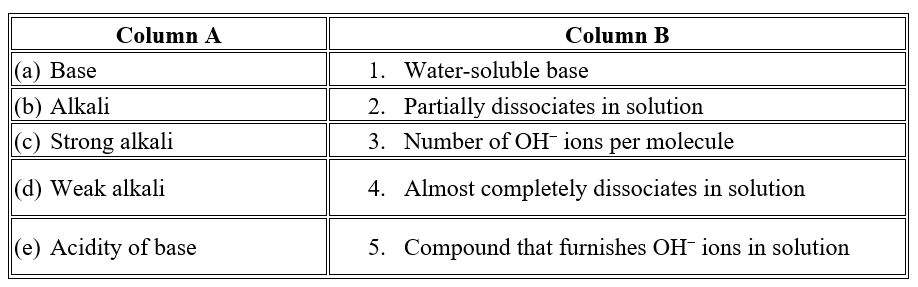
- Water-soluble bases are called _____________________________________________________.( Alkalis / Acids )
- The number of replaceable hydrogen atoms in an acid is known as its _______________________________________.( Basicity / Acidity )
- A base that furnishes one OH- ion per molecule is called a _______________________________________ base.( Monoacidic / Diacidic )
- The pH scale ranges from _______________________________________ to 14.( 0 / 7 )
- A pH value of 7 is considered _______________________________________.( Acidic / Neutral )
- Salts formed by the partial replacement of ionisable hydrogen atoms of an acid are called _______________________________________ salts.( Normal / Acid )
- The process of neutralization involves the reaction between an _______________________________________ and a base.( Acid / Alkali )
- Acid-base indicators are normally weak organic _______________________________________ and bases.( Acids / Salts )
- Universal Indicator is a mixture of sodium salt of _______________________________________ and other indicators.( Phenolphthalein / Methyl red )
SECTION C
- The correct order of increasing basic strength of the hydroxides is:
(a) KOH < NaOH < LiOH
(b) LiOH < NaOH < KOH
(c) NaOH < LiOH < KOH
(d) KOH < LiOH < NaOH - The correct order of increasing pH value of the solutions is:
(a) HCl < CH3COOH < NaOH
(b) NaOH < CH3COOH < HCl
(c) CH3COOH < HCl < NaOH
(d) HCl < NaOH < CH3COOH - The correct order of increasing acid strength of the acids is:
(a) CH3COOH < HNO3 < HCl
(b) HCl < HNO3 < CH3COOH
(c) HNO3 < CH3COOH < HCl
(d) CH3COOH < HCl < HNO3 - The correct order of increasing thermal stability of the carbonates is:
(a) Na2CO3 < K2CO3 < CuCO3
(b) CuCO3 < Na2CO3 < K2CO3
(c) K2CO3 < Na2CO3 < CuCO3
(d) Na2CO3 < CuCO3 < K2CO3 - The correct order of increasing solubility of the salts in water is:
(a) PbCl2 < AgCl < NaCl
(b) NaCl < AgCl < PbCl2
(c) AgCl < PbCl2 < NaCl
(d) PbCl2 < NaCl < AgCl - The correct order of increasing volatility of the acids is:
(a) HCl < CH3COOH < HNO3
(b) CH3COOH < HCl < HNO3
(c) HNO3 < HCl < CH3COOH
(d) HCl < HNO3 < CH3COOH
SECTION D
- Rohan added a few drops of silver nitrate solution to a sodium chloride solution in the lab. He observed a white precipitate being formed in the test tube. On addition of dilute nitric acid to the white precipitate and mixing it, he observed that the precipitate disappeared.
(a) Name the white precipitate.
(b) Write a balanced chemical equation for the reaction between dilute nitric acid and the white precipitate.
(c) Name the gas evolved in the above reaction. - Aarav prepared a sodium carbonate solution in the lab and added a few drops of sulphuric acid to it. He observed a colourless gas being evolved. On passing the gas through a solution of calcium hydroxide, he observed that the solution turned milky.
(a) Name the colourless gas evolved.
(b) Write a balanced chemical equation for the reaction between sulphuric acid and sodium carbonate.
(c) Name the compound responsible for the milky appearance. - Kunal added a few drops of barium chloride solution to a sodium sulphate solution in the lab. He observed a white precipitate being formed in the test tube. On addition of dilute hydrochloric acid to the white precipitate and mixing it, he observed that the precipitate disappeared.
(a) Name the white precipitate.
(b) Write a balanced chemical equation for the reaction between dilute hydrochloric acid and the white precipitate.
(c) Name the gas evolved in the above reaction. - Ishaan prepared a zinc sulphide solution in the lab and added a few drops of dilute sulphuric acid to it. He observed a colourless gas being evolved. On passing the gas through a solution of lead acetate, he observed that the solution turned black.
(a) Name the colourless gas evolved.
(b) Write a balanced chemical equation for the reaction between sulphuric acid and zinc sulphide.
(c) Name the compound responsible for the black appearance. - Siddharth added a few drops of silver nitrate solution to a sodium phosphate solution in the lab. He observed a yellow precipitate being formed in the test tube. On addition of dilute nitric acid to the yellow precipitate and mixing it, he observed that the precipitate disappeared.
(a) Name the yellow precipitate.
(b) Write a balanced chemical equation for the reaction between dilute nitric acid and the yellow precipitate.
(c) Name the gas evolved in the above reaction. - Riya was given an unknown salt solution. On adding dilute hydrochloric acid to the solution, a brisk effervescence was observed, and the gas produced turned lime water milky.
(a) Name the likely anion present in the unknown salt.
(b) Write a balanced chemical equation for the reaction between the unknown salt and dilute hydrochloric acid.
(c) If the unknown salt was a sodium salt, write the chemical formula of the salt. - Aryan performed an experiment where they added a few drops of sodium hydroxide solution to a solution of iron(III) chloride. They observed a reddish-brown precipitate.
(a) Write the chemical formula of the reddish-brown precipitate.
(b) Write a balanced chemical equation for the reaction that occurred.
(c) If dilute sulfuric acid is added to the reddish-brown precipitate, what will be observed? - Neha added a piece of magnesium ribbon to dilute sulfuric acid in a test tube. They observed the evolution of a colorless gas, which produced a “pop” sound when a burning splint was brought near it.
(a) Name the gas evolved in the reaction.
(b) Write a balanced chemical equation for the reaction between magnesium and sulfuric acid.
(c) What type of reaction is this? - Vikram mixed a solution of lead(II) nitrate with a solution of sodium chloride. A white precipitate was formed.
(a) Name the white precipitate formed.
(b) Write a balanced chemical equation for the reaction that occurred.
(c) If the white precipitate is heated, what happens to it? - Priya was given a solution of an unknown base. On adding ammonium chloride solution and heating the mixture, a pungent gas was evolved which turned red litmus paper blue.
(a) Name the gas evolved.
(b) Name a likely cation present in the unknown base.
(c) Write a balanced chemical equation for the reaction between ammonium chloride and a possible unknown base. - A student added dilute hydrochloric acid to a test tube containing sodium carbonate powder. He observed effervescence due to the evolution of a gas. The gas was passed through lime water, and it turned milky.
(a) Name the gas evolved.
(b) Write the balanced chemical equation for the reaction between sodium carbonate and hydrochloric acid.
(c) Explain why the lime water turns milky. - In a laboratory experiment, a student mixed lead nitrate solution with potassium iodide solution. He observed the formation of a yellow precipitate.
(a) Identify the yellow precipitate formed in the reaction.
(b) Write a balanced chemical equation for this reaction.
(c) What type of reaction is this? - A student added manganese dioxide (MnO₂) to a solution of hydrogen peroxide (H₂O₂). He observed bubbles forming in the solution. When a glowing splint was introduced into the test tube, it reignited.
(a) Name the gas responsible for reigniting the glowing splint.
(b) Write the balanced chemical equation for the decomposition of hydrogen peroxide in the presence of manganese dioxide.
(c) What is the role of manganese dioxide in this reaction? - A student placed a small piece of zinc metal into a test tube containing dilute sulfuric acid. He observed bubbling in the test tube. The evolved gas was collected and tested with a burning splint, which produced a ‘pop’ sound.
(a) Name the gas evolved in the reaction.
(b) Write a balanced chemical equation for the reaction.
(c) What type of reaction is this? - A student dipped an iron nail into a blue solution of copper sulfate and left it undisturbed for a while. He observed that the blue color of the solution gradually faded, and a reddish-brown deposit formed on the iron nail.
(a) What is the reddish-brown deposit formed on the iron nail?
(b) Write the balanced chemical equation for the reaction.
(c) What type of reaction is this?
SECTION E
Question 1
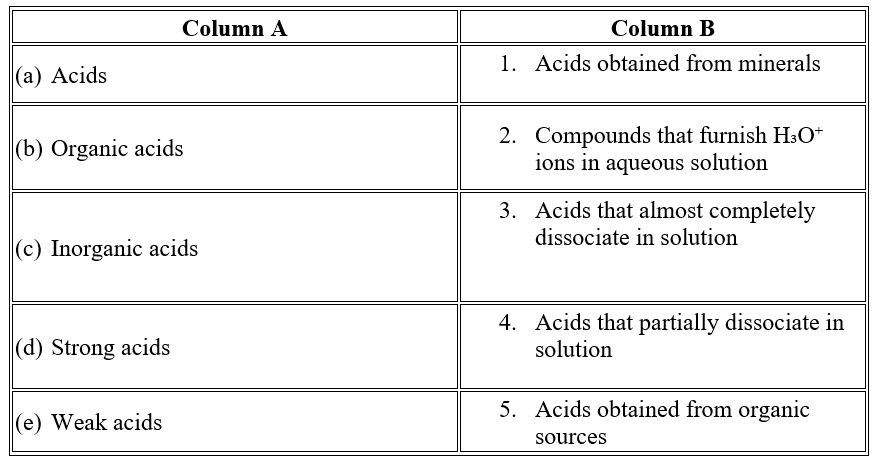
Question 2

Question 3
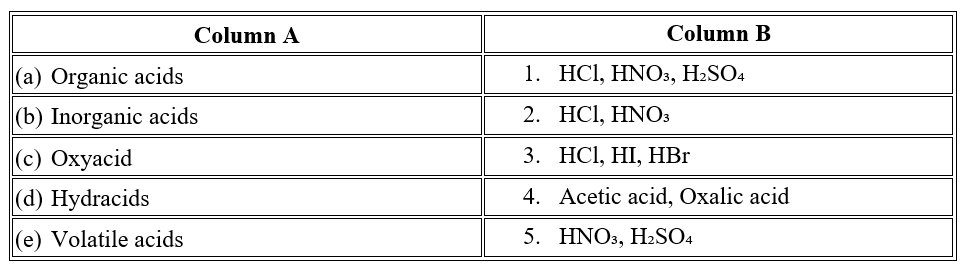
Question 4
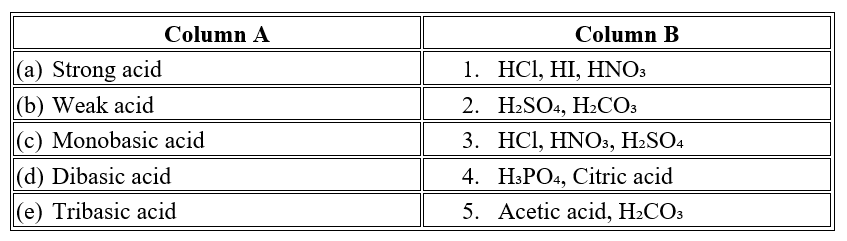
Question 5

Question 6
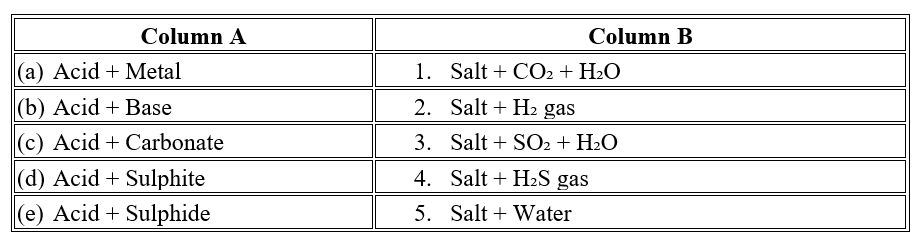
Question 7
Question 8
Question 9
Question 10

SECTION F
- An element Y forms a compound YCl4. This compound is a good conductor of electricity and can be reduced to its metal only by electrolysis.
(a) Write the equation for the reaction formed when the compound (YCl4) combines with water.
(b) How many valence electrons are present in the outermost shell of Y?
(c) Will element Y undergo oxidation or reduction? - A metal M forms an oxide MO2. This oxide is insoluble in water and can be reduced to its metal by heating with carbon.
(a) Write the equation for the reaction formed when the oxide (MO2) combines with hydrochloric acid.
(b) What is the oxidation state of M in the oxide?
(c) Will element M undergo oxidation or reduction? - An element Z forms a compound ZO3. This compound is a strong oxidizing agent and can be reduced to its metal only by electrolysis.
(a) Write the equation for the reaction formed when the compound (ZO3) combines with sulfuric acid.
(b) How many valence electrons are present in the outermost shell of Z?
(c) Will element Z undergo oxidation or reduction? - A metal N forms a sulfide NS2. This sulfide is insoluble in water and can be reduced to its metal by heating with hydrogen.
(a) Write the equation for the reaction formed when the sulfide (NS2) combines with nitric acid.
(b) What is the oxidation state of N in the sulfide?
(c) Will element N undergo oxidation or reduction? - An element P forms a compound PCl5. This compound is a strong oxidizing agent and can be reduced to its metal only by electrolysis.
(a) Write the equation for the reaction formed when the compound (PCl5) combines with water.
(b) How many valence electrons are present in the outermost shell of P?
(c) Will element P undergo oxidation or reduction? - A metal Q forms an oxide QO. This oxide is insoluble in water and can be reduced to its metal by heating with carbon.
(a) Write the equation for the reaction formed when the oxide (QO) combines with hydrochloric acid.
(b) What is the oxidation state of Q in the oxide?
(c) Will element Q undergo oxidation or reduction? - An element R forms a compound R2O7. This compound is a strong oxidizing agent and can be reduced to its metal only by electrolysis.
(a) Write the equation for the reaction formed when the compound (R2O7) combines with sulfuric acid.
(b) How many valence electrons are present in the outermost shell of R?
(c) Will element R undergo oxidation or reduction? - A metal S forms a sulfide S2S3. This sulfide is insoluble in water and can be reduced to its metal by heating with hydrogen.
(a) Write the equation for the reaction formed when the sulfide (S2S3) combines with nitric acid.
(b) What is the oxidation state of S in the sulfide?
(c) Will element S undergo oxidation or reduction? - An element T forms a compound TCl3. This compound is a strong oxidizing agent and can be reduced to its metal only by electrolysis.
(a) Write the equation for the reaction formed when the compound (TCl3) combines with water.
(b) How many valence electrons are present in the outermost shell of T?
(c) Will element T undergo oxidation or reduction? - A metal U forms an oxide UO3. This oxide is insoluble in water and can be reduced to its metal by heating with carbon.
(a) Write the equation for the reaction formed when the oxide (UO3) combines with hydrochloric acid.
(b) What is the oxidation state of U in the oxide?
(c) Will element U undergo oxidation or reduction? - An element Y reacts with chlorine to form a chloride YCl₂. This chloride dissolves in water to form an acidic solution.
(a) Write the balanced chemical equation for the reaction between YCl₂ and water.
(b) Identify whether element Y is a metal or a non-metal.
(c) Will element Y gain or lose electrons in a chemical reaction? - An element Z reacts with sulfur to form Z₂S₃, which is an ionic compound. It is found in nature as a mineral and can conduct electricity in molten form.
(a) Write the chemical equation for the reaction between Z₂S₃ and dilute hydrochloric acid.
(b) How many valence electrons does Z have?
(c) Which group of the periodic table is element Z likely to belong to? - A certain element A reacts with oxygen to form A₂O₅. This oxide is amphoteric in nature.
(a) Write balanced chemical equations for the reaction of A₂O₅ with hydrochloric acid and sodium hydroxide.
(b) Identify whether element A is a metal, non-metal, or metalloid.
(c) What property of A₂O₅ makes it amphoteric? - An unknown element B forms a basic oxide BO, which reacts with water to form a strong base.
(a) Write the equation for the reaction of BO with water.
(b) What type of bonding is present in BO?
(c) Would B be located on the left or right side of the periodic table? - A metal M forms a carbonate MCO₃, which decomposes upon heating to form metal oxide MO and carbon dioxide gas.
(a) Write the balanced equation for the decomposition reaction.
(b) Predict the solubility of MCO₃ in water and explain.
(c) How can the presence of CO₂ be confirmed experimentally? - An element C forms an acidic oxide CO₂, which reacts with a strong base to form a salt and water.
(a) Write the balanced equation for the reaction between CO₂ and sodium hydroxide.
(b) What type of bonding is present in CO₂?
(c) Would C be classified as a metal or a non-metal? - A metal D is extracted from its ore using carbon reduction. Its oxide is not amphoteric.
(a) Write the general equation for the reduction of D₂O₃ using carbon.
(b) Why is carbon reduction used instead of electrolysis?
(c) Would D be more reactive or less reactive than sodium? - An unknown element E reacts with halogens to form EX₂-type compounds. These compounds dissolve in water to give a neutral solution.
(a) Write a general equation for the reaction of E with chlorine.
(b) What does the neutrality of the aqueous solution suggest about the nature of EX₂?
(c) How many valence electrons does E have? - A certain element F is stored in oil because it reacts violently with water, forming an alkali and hydrogen gas.
(a) Write the chemical equation for the reaction of F with water.
(b) Identify the group in which F belongs.
(c) What observation would confirm the presence of hydrogen gas in this reaction? - An element G reacts with oxygen to form G₂O, which is highly soluble in water and forms a strong alkaline solution.
(a) Write the equation for the reaction of G₂O with water.
(b) What would be the expected pH of the resulting solution?
(c) Would G₂O conduct electricity in molten state? Explain. - An element Y forms a chloride YCl2 that is soluble in water. The element reacts with water to produce a basic solution and hydrogen gas.
(a) Write the balanced chemical equation for the reaction of Y with water.
(b) Predict the group of element Y in the periodic table.
(c) Will the oxide of Y be acidic or basic? - Element Z reacts with hydrogen to form a covalent compound ZH4. This compound is a gas at room temperature and is a poor conductor of electricity.
(a) Write the chemical formula of the oxide formed by Z.
(b) Determine the number of valence electrons in element Z.
(c) Predict the type of bonding in the chloride of Z. - An element P forms an oxide P2O5, which dissolves in water to form an acidic solution. The element P is a non-metal.
(a) Write the balanced chemical equation for the reaction of P2O5 with water.
(b) Predict the group of element P in the periodic table.
(c) Will P gain or lose electrons when forming ions? - Element Q forms a compound QCl that is a solid at room temperature and conducts electricity when molten. The element reacts vigorously with water.
(a) Write the balanced chemical equation for the reaction of Q with water.
(b) Predict the group of element Q in the periodic table.
(c) What type of bond is formed in QCl? - Element R forms a hydride RH3 that is a gas at room temperature. The oxide of R is acidic in nature.
(a) Write the chemical formula of the oxide formed by R.
(b) Determine the number of valence electrons in element R.
(c) Predict the type of bonding in RH3. - Element S forms a sulfate S2(SO4)3. The oxide of S is amphoteric in nature.
(a) Write the balanced chemical equation for the reaction of the oxide of S with hydrochloric acid.
(b) Predict the group of element S in the periodic table.
(c) Will S form positive or negative ions? - Element T forms a compound TBr2 that is used in photography. The element reacts with oxygen to form a basic oxide.
(a) Write the balanced chemical equation for the reaction of T with oxygen.
(b) Predict the group of element T in the periodic table.
(c) What type of bond is formed in TBr2? - Element U forms a chloride UCl that is a gas at room temperature. The element reacts with hydrogen to form a covalent compound.
(a) Write the chemical formula of the hydride formed by U.
(b) Determine the number of valence electrons in element U.
(c) Will U form positive or negative ions? - Element V forms an oxide VO2 that is a colored solid. The element is a transition metal.
(a) Write the balanced chemical equation for the reaction of VO2 with sulfuric acid.
(b) Predict the possible oxidation states of V.
(c) Will V form acidic or basic oxides? - Element W forms a compound W2O that is a strong reducing agent. The element reacts vigorously with water.
(a) Write the balanced chemical equation for the reaction of W with water.
(b) Predict the group of element W in the periodic table.
(c) What type of bond is formed in W2O?
SECTION G
Passage 1:
The pH scale is a measure of the acidity or alkalinity of a solution. It ranges from 0 to 14, with pH 7 being neutral. A pH value less than 7 indicates an acidic solution, while a pH greater than 7 indicates a basic (alkaline) solution. The pH of a solution depends on the concentration of hydrogen ions (H⁺). Strong acids, such as hydrochloric acid (HCl), have very low pH values, while strong bases, such as sodium hydroxide (NaOH), have very high pH values. The pH of natural substances, like soil and water, is important for plant and animal life. Even small changes in pH can affect biological systems and chemical reactions.
Questions:
- What does a pH value of 7 indicate?
a) Acidic solution
b) Basic solution
c) Neutral solution
d) Strong acid - If a solution has a pH of 3, it is considered:
a) Strongly acidic
b) Weakly acidic
c) Neutral
d) Basic - Which factor primarily determines the pH of a solution?
a) The color of the solution
b) The concentration of hydrogen ions (H⁺)
c) The temperature of the solution
d) The volume of the solution - What happens to the pH when an acid is added to water?
a) It increases
b) It remains the same
c) It decreases
d) It becomes neutral
Passage 2:
The pH of different substances varies widely. Pure water has a pH of 7, making it neutral. Lemon juice, with a pH of around 2, is highly acidic, while household ammonia, with a pH of about 11, is strongly basic. Human blood maintains a pH around 7.4, which is slightly basic and crucial for bodily functions. The pH of rainwater is normally around 5.6 due to dissolved carbon dioxide, but acid rain has a pH below 5 due to pollutants like sulfur dioxide (SO₂) and nitrogen oxides (NOₓ). Acid rain can damage buildings, harm aquatic life, and affect soil quality.
Questions:
- What is the approximate pH of pure water?
a) 2
b) 7
c) 11
d) 5 - Why is human blood slightly basic?
a) It contains hydrochloric acid
b) It has a high concentration of H⁺ ions
c) It helps maintain body functions
d) It is affected by acid rain - What causes acid rain?
a) Carbon dioxide alone
b) Sulfur dioxide (SO₂) and nitrogen oxides (NOₓ)
c) Ammonia and baking soda
d) Oxygen and nitrogen - Which of the following substances is highly acidic?
a) Ammonia
b) Lemon juice
c) Soap solution
d) Blood
Passage 3
The pH scale is used to measure the acidity or basicity of a solution. The pH scale ranges from 0 to 14, with a pH of 7 being neutral. A pH less than 7 is acidic, while a pH greater than 7 is basic. Substances with a high pH, such as soap, can irritate the skin.
Questions
- What is the range of the pH scale?
- What is the pH of a neutral solution?
- What type of solution has a pH less than 7?
- Why can substances with high pH irritate the skin?
Passage 4
The pH of a solution can be measured using pH paper or a pH meter. pH paper changes color depending on the pH of the solution. A pH meter provides a more accurate reading of the pH. The pH of common substances varies widely, with lemon juice having a pH of around 2 and baking soda having a pH of around 8.
Questions
- What are two methods of measuring pH?
- How does pH paper work?
- What is the approximate pH of lemon juice?
- What is the approximate pH of baking soda?
Passage 5
Maintaining the proper pH is crucial in various industries, such as agriculture and medicine. In agriculture, the pH of the soil affects the growth of crops. In medicine, the pH of blood must be maintained within a narrow range to ensure proper bodily functions. A pH imbalance can lead to various health problems.
Questions
- Why is maintaining proper pH important in agriculture?
- What is the importance of pH in medicine?
- What can happen if the pH of blood becomes imbalanced?
Passage 6
Acids are substances that donate H+ ions in solution. When acids react with metals, they produce hydrogen gas and a salt. Acids also turn blue litmus paper red. Additionally, acids have a pH level of less than 7.
Questions
- What ions do acids donate in solution?
- What is produced when acids react with metals?
- How do acids affect litmus paper?
- What is the pH range of acids?
Passage 7
Strong acids, such as hydrochloric acid (HCl) and sulfuric acid (H2SO4), completely dissociate in water to produce H+ ions. Weak acids, such as acetic acid (CH3COOH), only partially dissociate in water. Acids also have the property of neutralizing bases.
Questions
- What type of acids completely dissociate in water?
- Give an example of a weak acid.
- What happens when acids react with bases?
Passage 8
Acids have various physical and chemical properties. They can be corrosive and have a sour taste. Acids can also react with carbonates to produce carbon dioxide gas. Furthermore, acids can be classified as organic or inorganic.
Questions
- What is the typical taste of acids?
- What gas is produced when acids react with carbonates?
- How can acids be classified?
SECTION H
Question 1
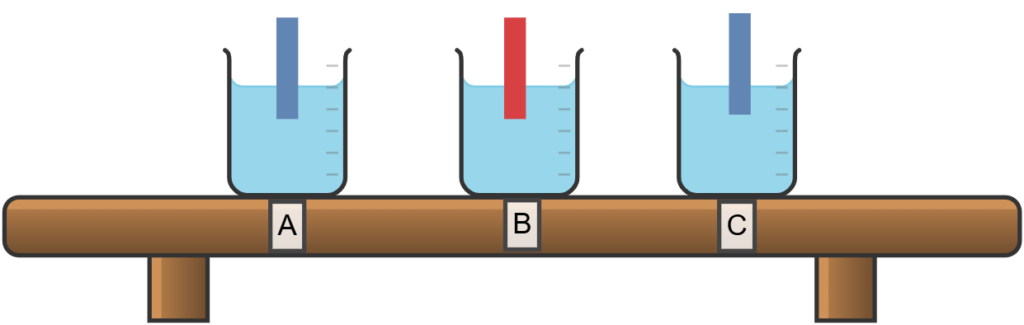
Three unknown solutions, labeled A, B, and C, were tested using litmus paper. Solution B caused blue litmus paper to turn red. When solution A was reacted with zinc metal and heated, a gas was produced that was identified as hydrogen. Solution C produced no noticeable change in the litmus paper and with Z. Classify the solutions as- Acidic , basic and Salt solution.
Question 2

The pH scale has numerous practical applications in various aspects of our lives. For instance, in agriculture, determining the pH of soil is crucial for proper farming. This is because different crops have optimal pH ranges for growth, and maintaining the right pH ensures healthy plant development. In the human body, pH plays a critical role in digestive health. When excess hydrochloric acid is produced in the stomach, causing acidity, antacids are consumed to neutralize the excess acid and provide relief.
- Why is monitoring and adjusting soil pH crucial in agriculture?
- How do antacids alleviate stomach acidity?
- What is the effect of sugary foods on the pH in the mouth, and how does toothpaste counteract this effect?
- Why are different treatments used for bee and wasp stings?
- What is the purpose of using hair conditioner after shampooing?
- What type of acid is present in ant stings, and what type of substance is used to neutralize it?
Question 2

What is the nature of wasp and bee stings, and which household substances are used to treat them?
A) Wasp stings are acidic, bee stings are alkaline; vinegar for wasp, bicarbonate for bee
B) Wasp stings are alkaline, bee stings are acidic; vinegar for wasp, bicarbonate for bee
C) Both wasp and bee stings are acidic; vinegar for both
D) Both wasp and bee stings are alkaline; bicarbonate for both
Question 3

What happens to the pH of the mouth cavity after consuming sugar or food, and how does brushing teeth help prevent teeth decay?
A) pH increases, and brushing teeth removes food particles
B) pH decreases, creating an acidic medium that favors bacterial growth, and brushing teeth neutralizes the acid with alkali
C) pH remains neutral, and brushing teeth only removes plaque
D) pH increases, and brushing teeth has no effect on bacterial growth















