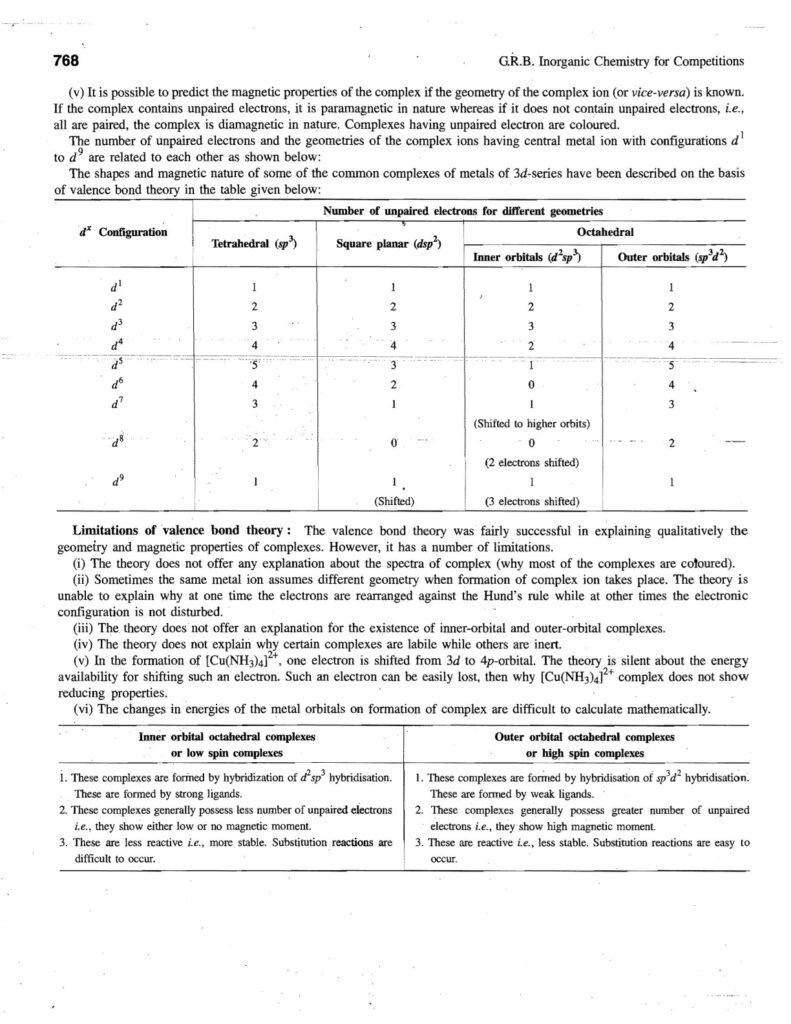
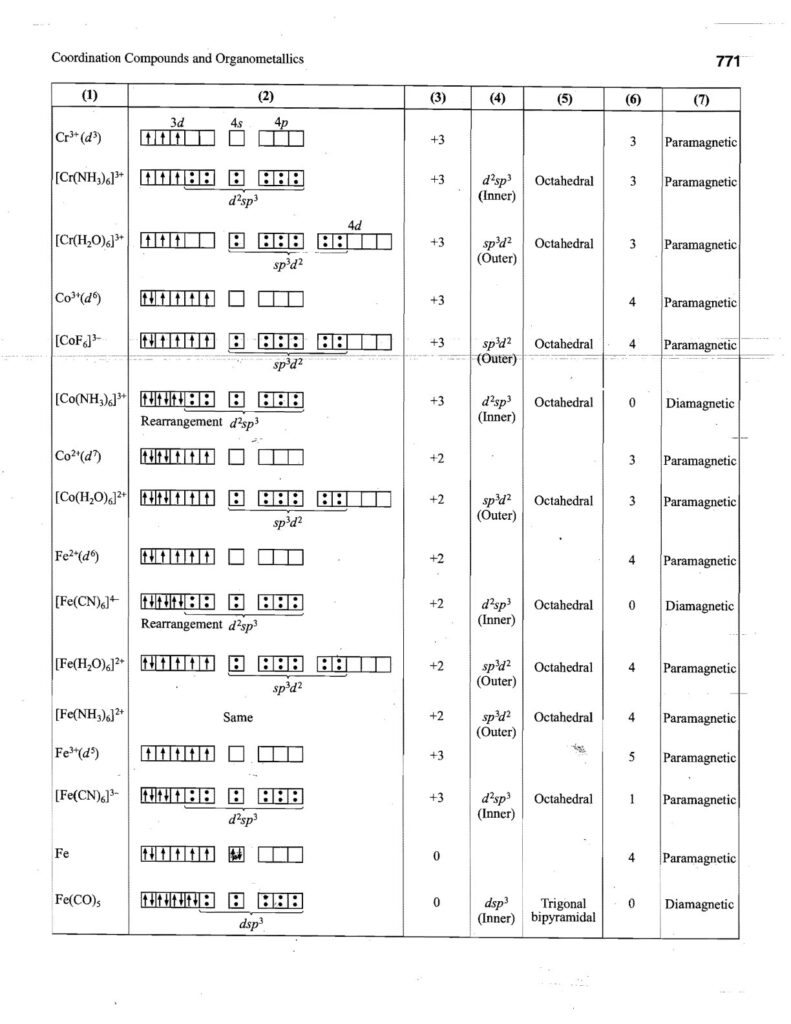
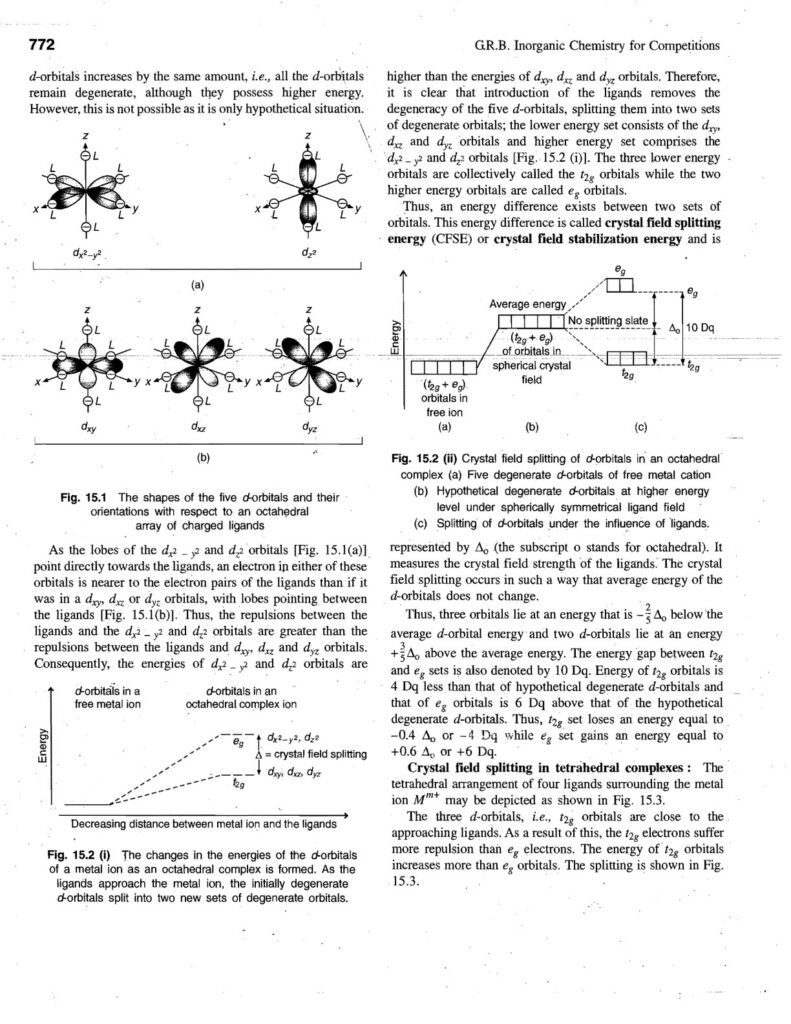
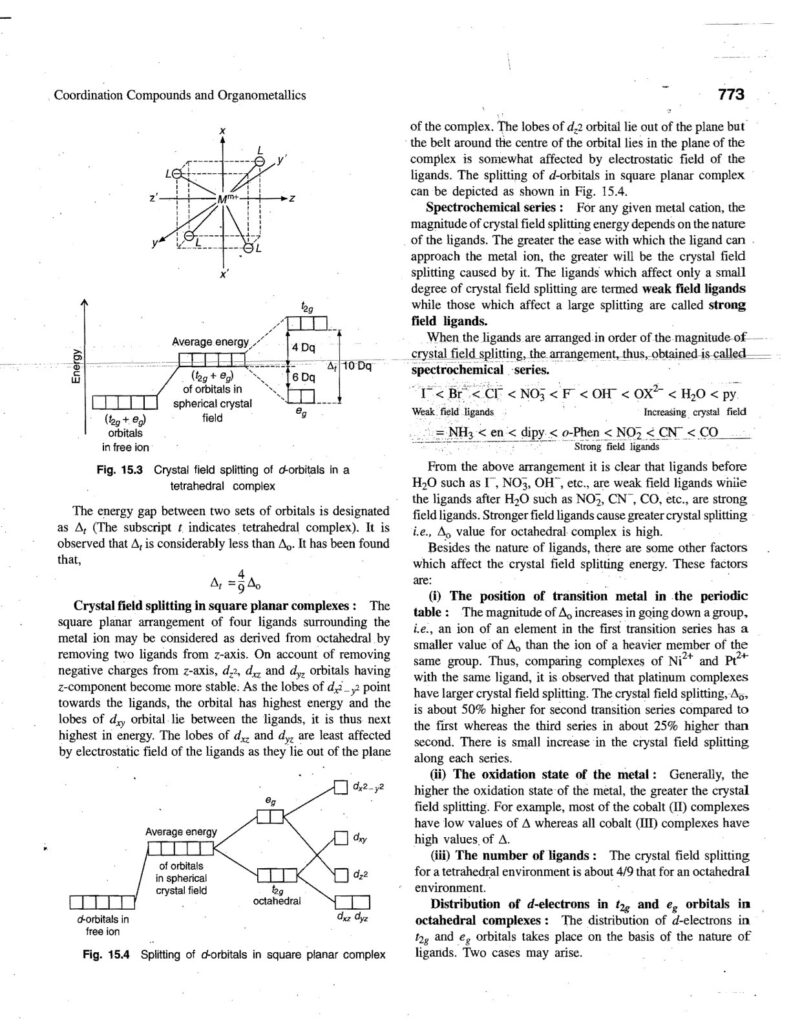
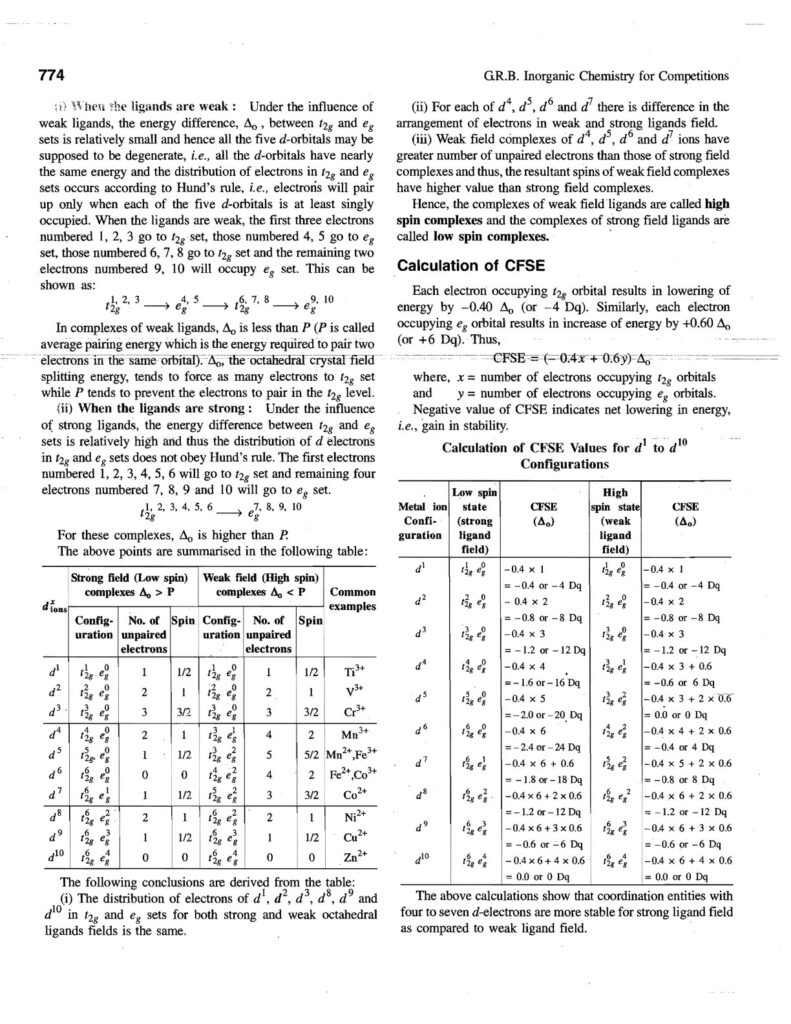
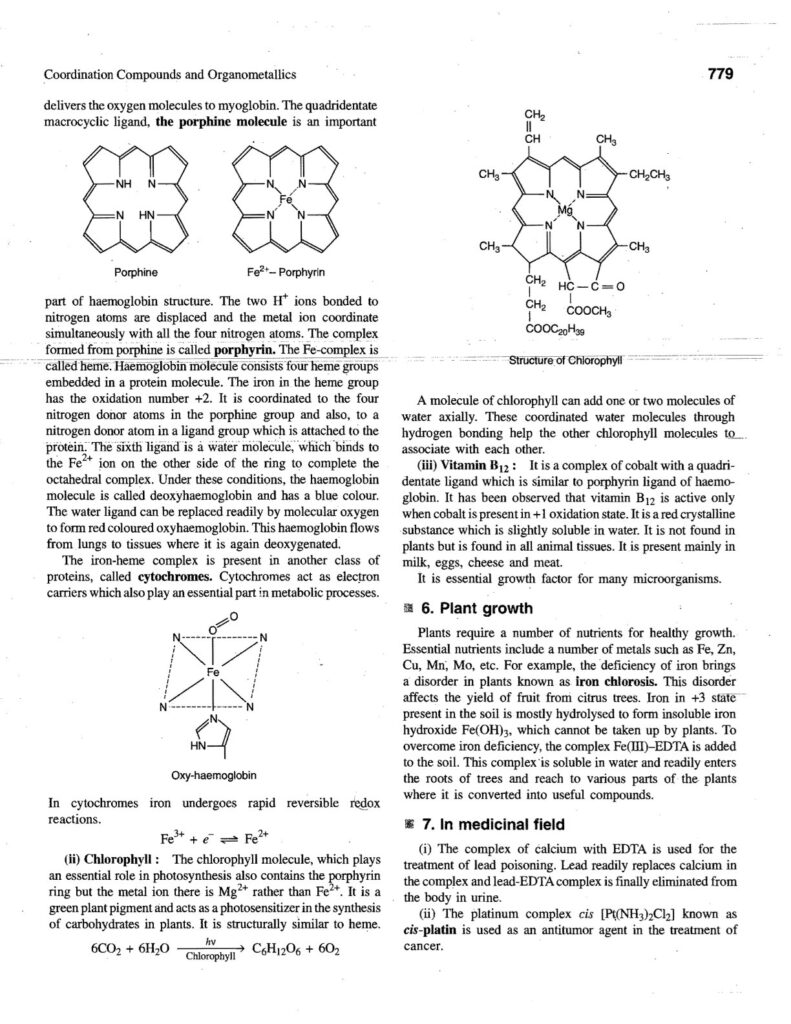
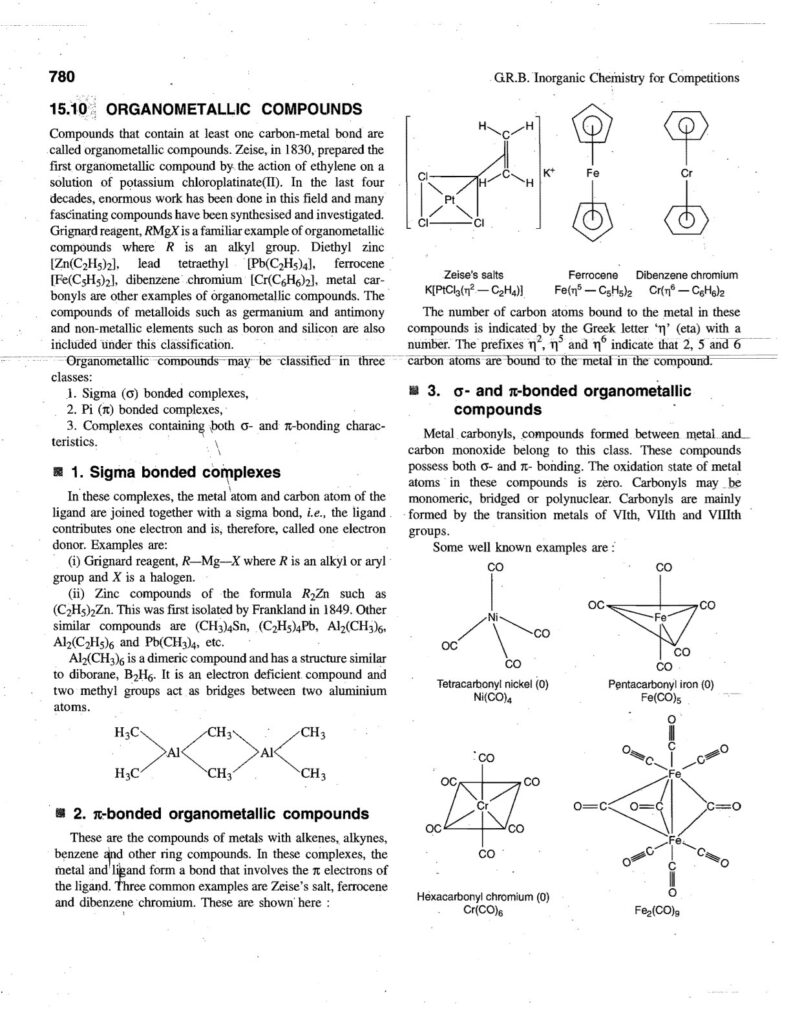
Coordination compounds, also known as complex compounds, are molecules or ions formed by the combination of a central metal atom or ion with a number of ligands. Here’s a note covering key aspects:
1. Definition and Formation:
- A coordination compound consists of a central metal atom or ion bonded to a surrounding array of molecules or ions called ligands.
- These bonds are typically coordinate covalent bonds, where the ligand donates a lone pair of electrons to the metal.
2. Key Components:
- Central Metal Atom/Ion:
- Usually a transition metal, which can exhibit multiple oxidation states and have vacant d-orbitals available for bonding.
- Acts as a Lewis acid (electron acceptor).
- Ligands:
- Molecules or ions that donate electron pairs to the central metal.
- Act as Lewis bases (electron donors).
- Can be neutral (e.g., H₂O, NH₃, CO) or charged (e.g., Cl⁻, CN⁻, OH⁻).
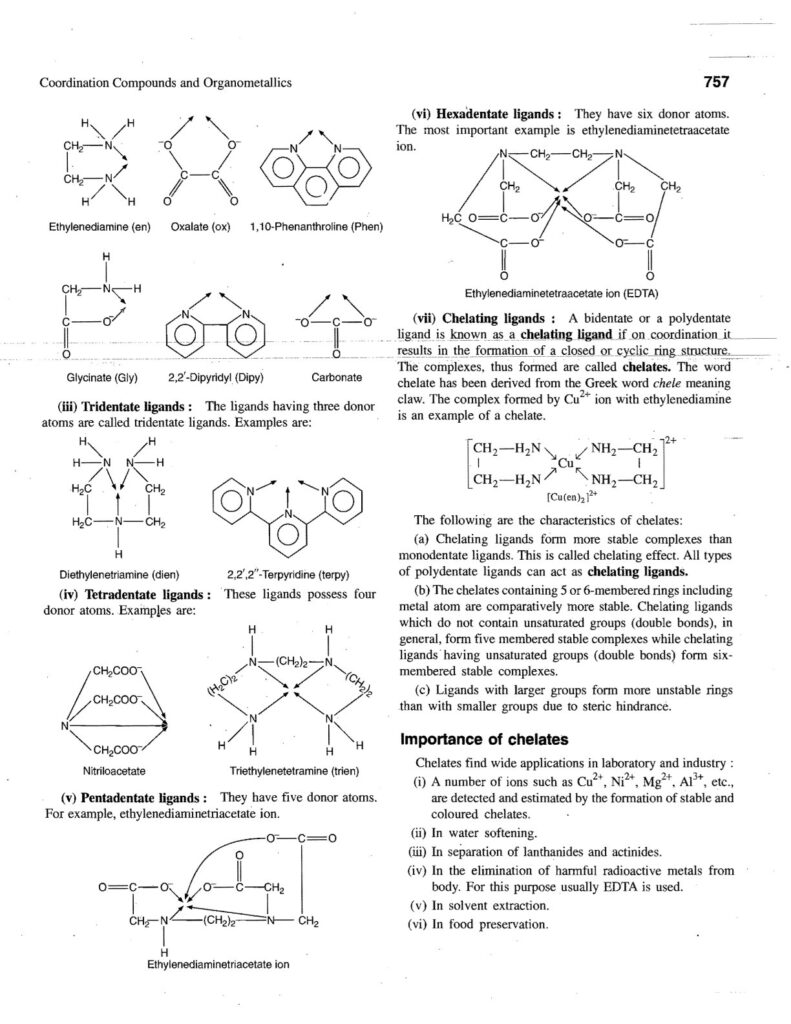
- Denticity: Refers to the number of donor atoms a ligand uses to bind to the central metal.
- Monodentate: One donor atom (e.g., Cl⁻, NH₃).
- Bidentate: Two donor atoms (e.g., ethylenediamine (en)).
- Polydentate: More than two donor atoms (e.g., EDTA).
- Chelating ligands: Bidentate and Polydentate ligands that form ring like structures with the metal.
- Coordination Sphere:
- The central metal atom/ion and its attached ligands enclosed in square brackets [ ].
- The coordination sphere behaves as a single unit.
- Counter Ions:
- Ions outside the coordination sphere that balance the charge of the complex ion.
- The number of ligand donor atoms directly bonded to the central metal atom.
- Determines the geometry of the complex.
- Common coordination numbers: 2, 4, and 6.
- Coordination number 2 is usually linear. Coordination number 4 can be tetrahedral or square planar. Coordination number 6 is usually octahedral.
4. Nomenclature:
- Specific rules are used to name coordination compounds, including:
- Naming the ligands alphabetically before the metal.
- Indicating the number of ligands using prefixes (di, tri, tetra, etc.).
- Specifying the oxidation state of the metal using Roman numerals in parentheses.
- Naming anionic ligands with an -o suffix(Chloro, cyano).
- Example: [Co(NH3)6]Cl3 is named hexaamminecobalt(III) chloride.
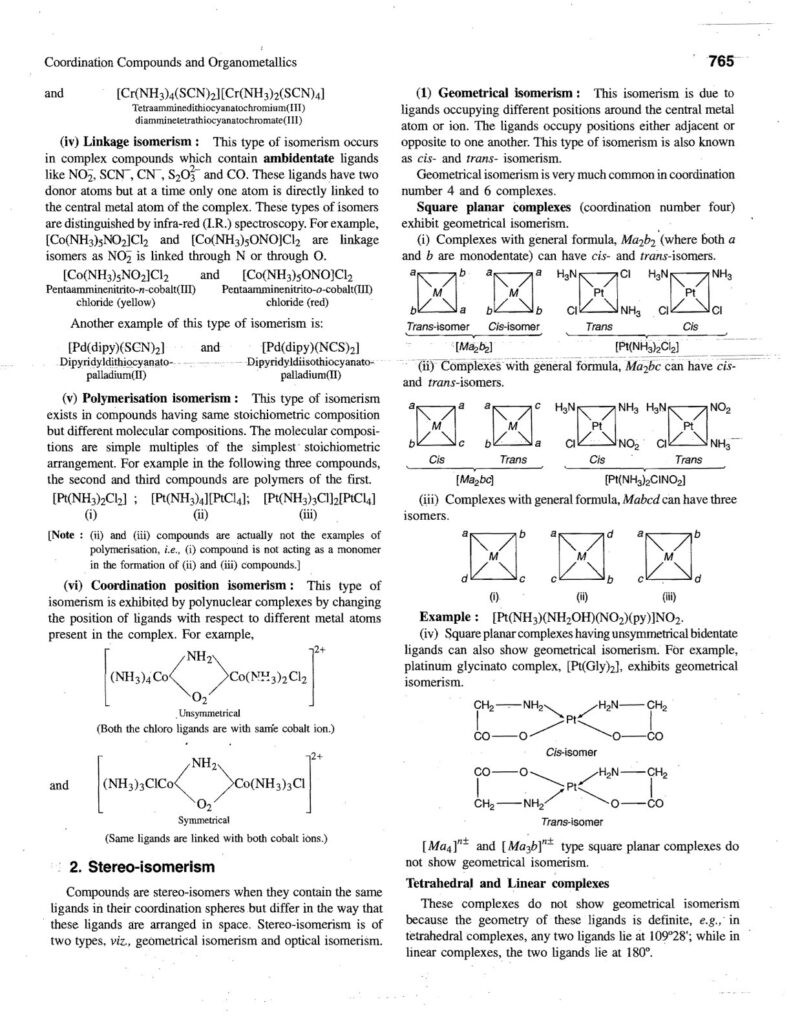
5. Isomerism:
- Coordination compounds can exhibit various types of isomerism:
- Structural Isomerism: Different connectivity.
- Ionization isomerism.
- Hydrate isomerism.
- Linkage isomerism.
- Coordination isomerism.
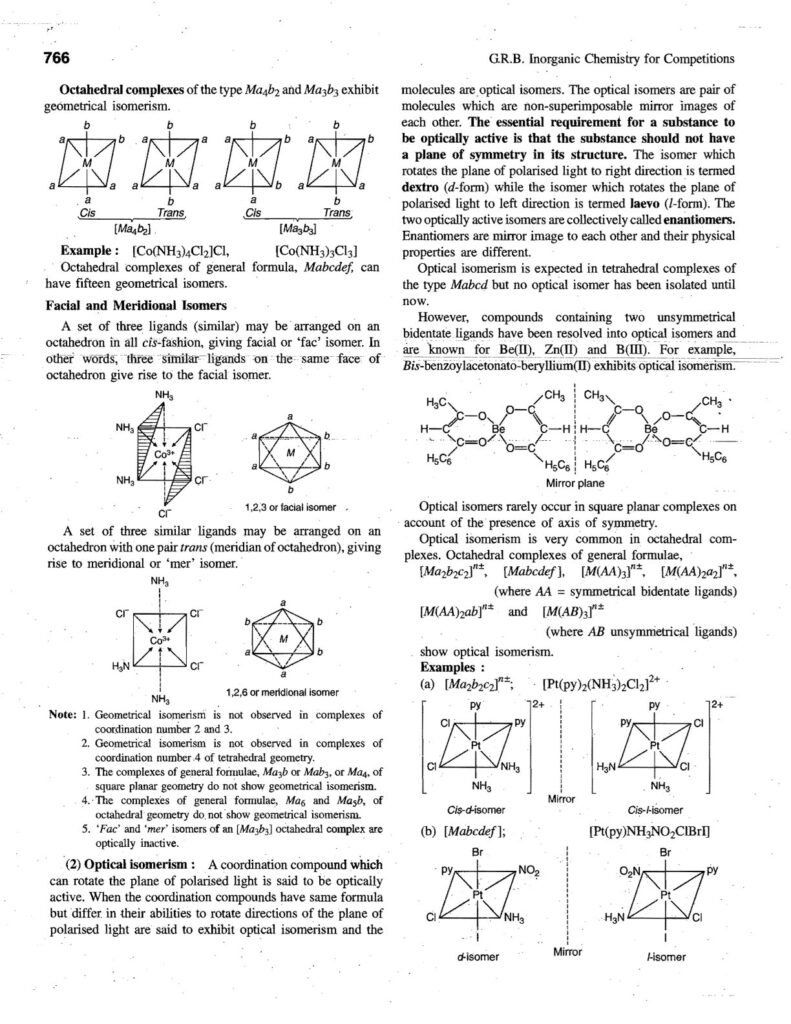
- Stereoisomerism: Same connectivity, different spatial arrangement.
- Geometric isomerism (cis-trans).
- Optical isomerism (enantiomers).
- Structural Isomerism: Different connectivity.
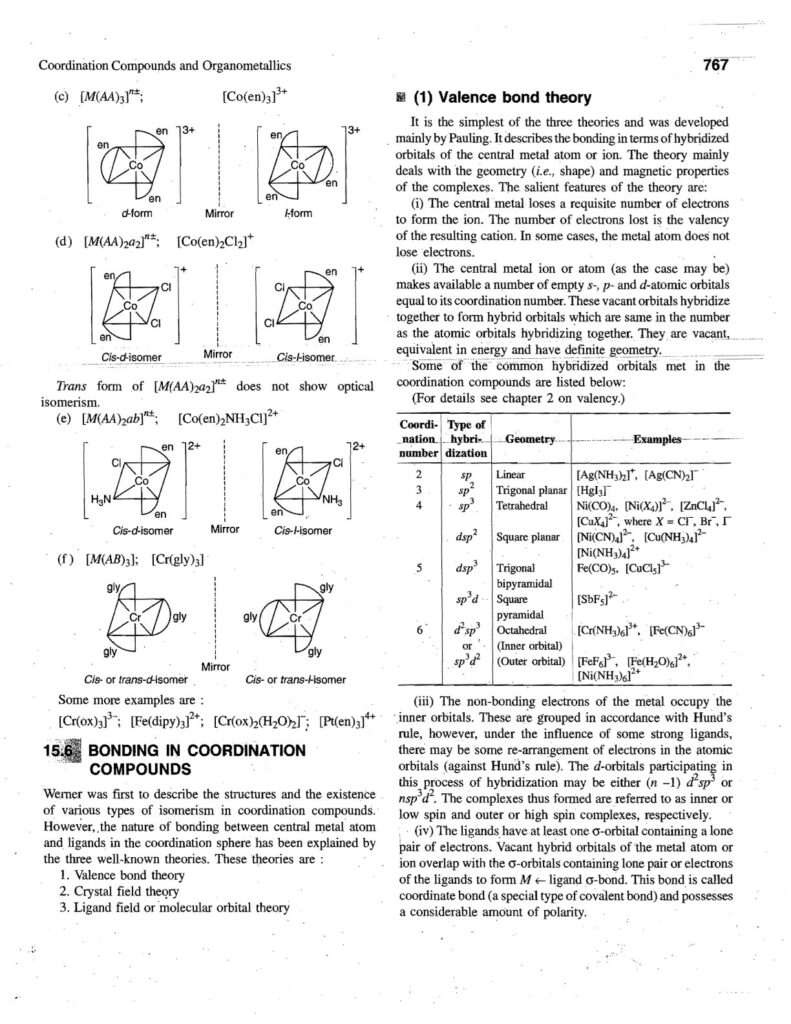
6. Bonding Theories:
- Valence Bond Theory (VBT): Explains bonding in terms of hybridization of metal orbitals.
- Crystal Field Theory (CFT): Explains bonding in terms of electrostatic interactions between the metal ion and ligands, and the splitting of d-orbitals.
- Ligand Field Theory (LFT): An advanced theory that combines aspects of VBT and CFT.
7. Applications:
- Catalysis: Many coordination compounds act as catalysts in industrial processes.
- Medicine: Used in chemotherapy (e.g., cisplatin), MRI contrast agents, and treatment of metal poisoning.
- Analytical Chemistry: Used in complexometric titrations and metal ion detection.
- Pigments and Dyes: Many coordination compounds are brightly colored and used as pigments.
- Extraction and purification of metals.
- Photography.