Equilibrium is a fundamental concept in chemistry that describes the state in which the concentrations of reactants and products in a chemical reaction remain constant over time. In other words, the rates of forward and reverse reactions are equal, and there is no net change in the concentrations of reactants and products.
In Class 11, you will delve into the world of chemical equilibrium, exploring the principles, laws, and applications that govern this crucial concept. Through these notes, you will gain a deeper understanding of equilibrium, including the equilibrium constant, Le Chatelier’s principle, and the factors that influence equilibrium.
Key Topics Covered:
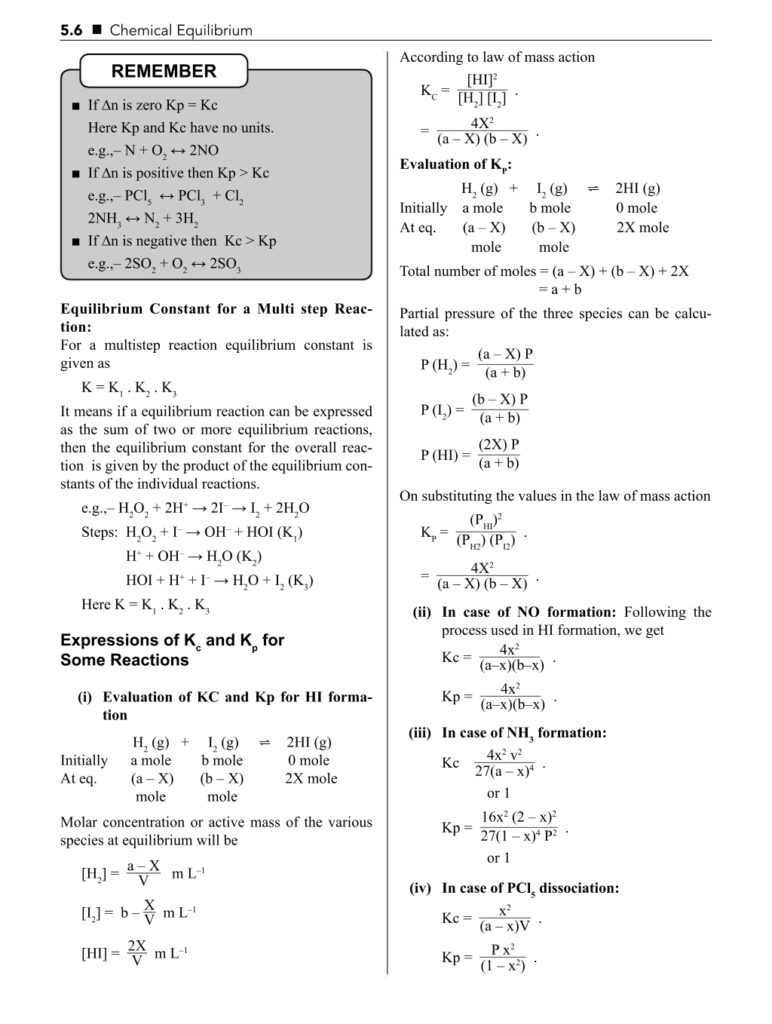
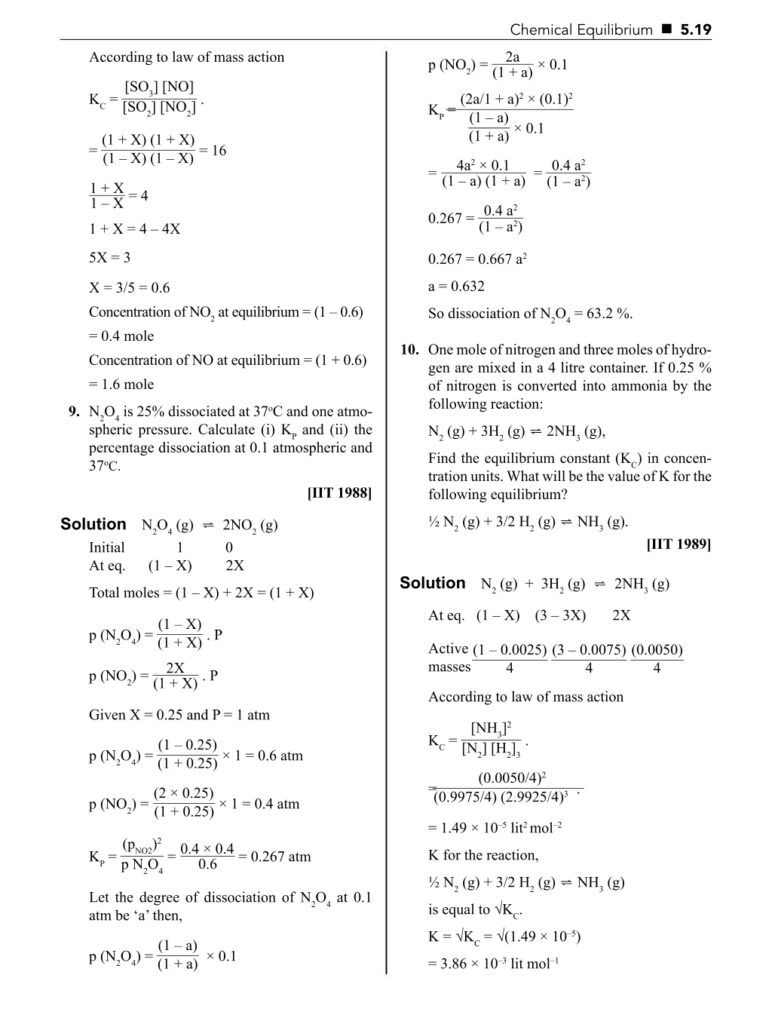
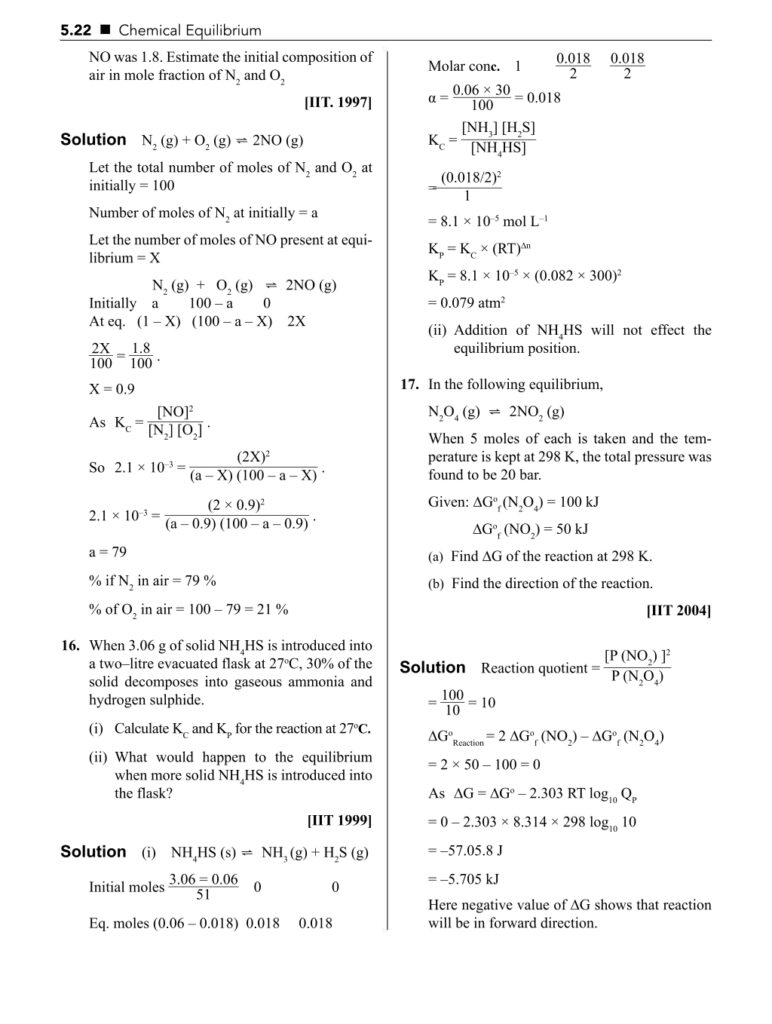
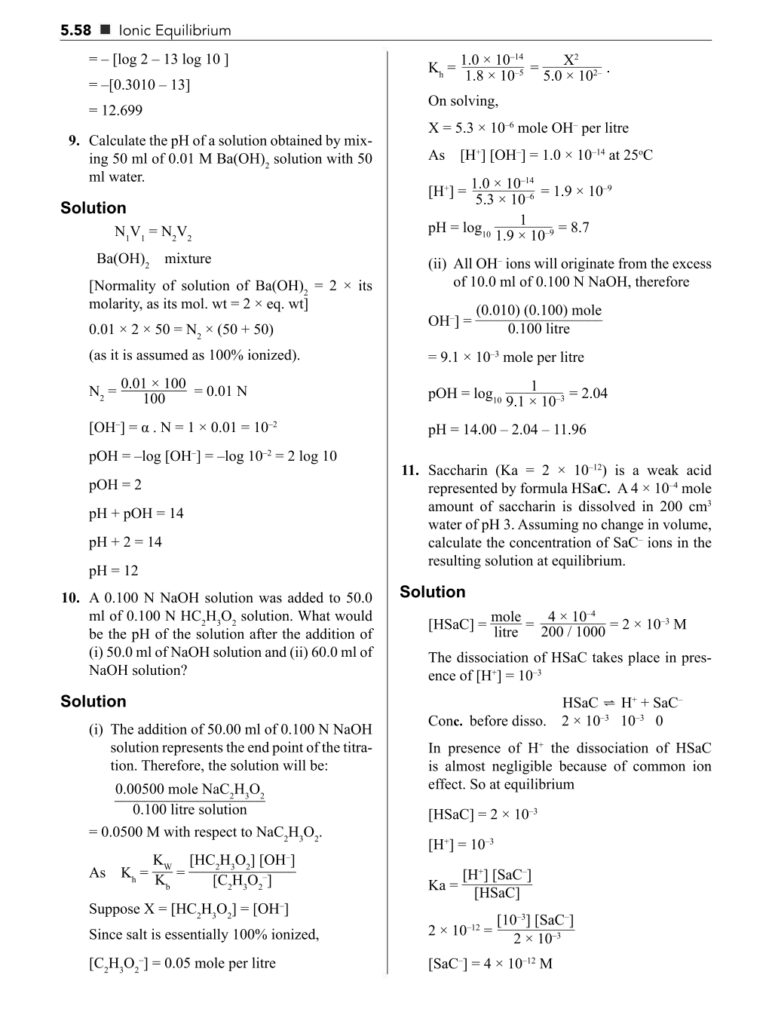
- Equilibrium constant (Kc and Kp)
- Law of mass action
- Le Chatelier’s principle
- Factors affecting equilibrium
- Applications of equilibrium
Get ready to explore the fascinating world of chemical equilibrium!