Synthesis:
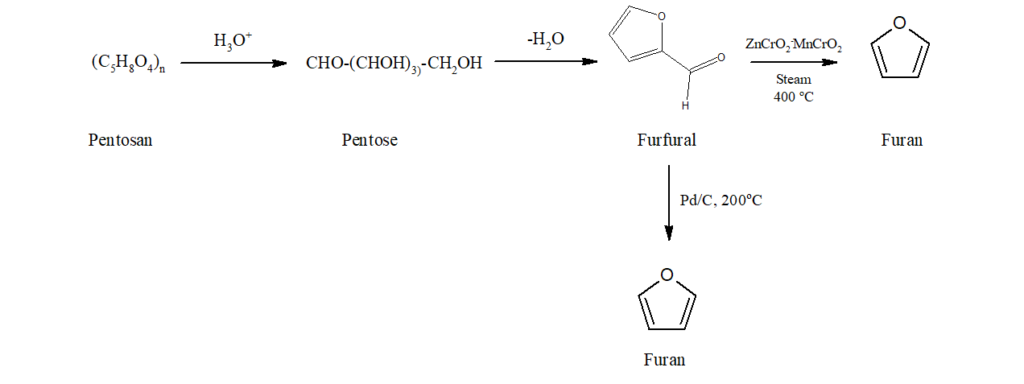
a) Oat hulls, rice hulls and corncobs contain pentosan which on treatment with hot hydrochloric acid gets converted into furfural. When furfural is decomposed in steam at 400oC in the presence of oxide catalyst, furan is obtained.
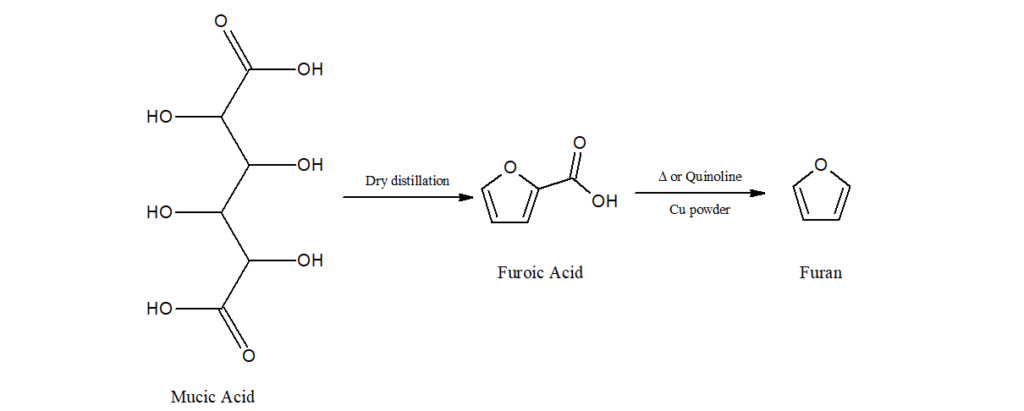
b) It can also be synthesized from mucic acid.
Reactivity:
Furan is a monocyclic planar molecule with (4n + 2)Π system.

Furan is less aromatic than thiophene and pyrrole , probably due to the strong electronegative character of the O atom, which draws the Π-cloud much towards itself .
Chemical properties:
a)Although furan is an aromatic compound, yet it shows a few properties of conjugated dienes.
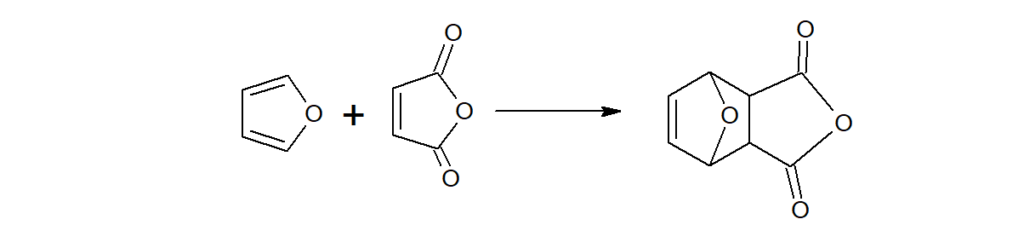
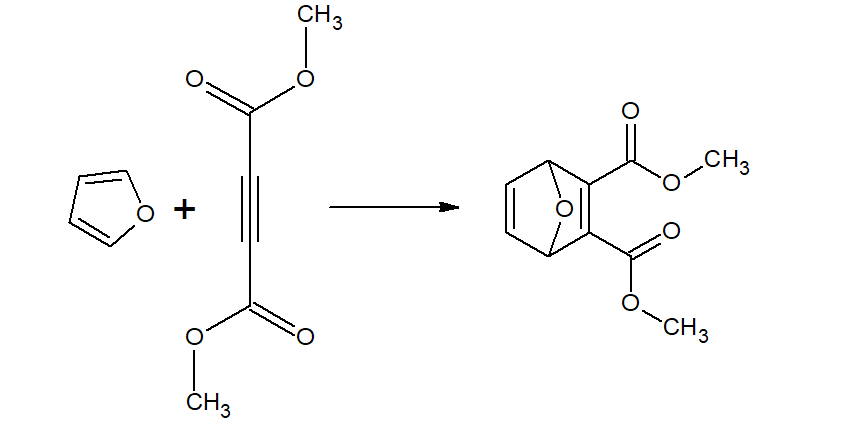
Furan undergoes Diel-Alder reaction with maleic anhydride.(which pyrrole and thiophene does not)
b)Furan is catalytically reduced to tetrahydrofuran.

c)Oxidation of furan gives succinaldehyde. In presence of acids, resinification occurs.

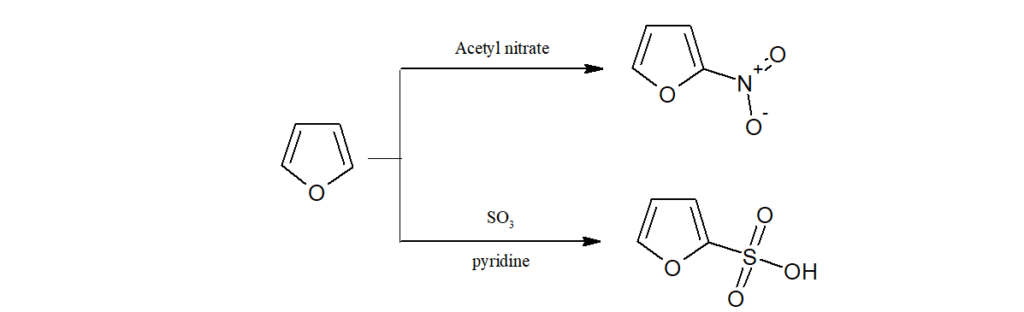
d)As furan is labile to the action of acids, nitration is done by acetyl nitrate, sulphonation by SO3/Pyridine.
e)As direct halogenations of furan gives polymer, it is prepared indirectly via mercuration or from furoic acid.

f)Friedel Craft alkylation of furan leads to polymerization. But it can be acylated in presence of SnCl4 catalyst(As AlCl3 attack the furan it is not used). It can also be acylated via mercuration.

g)Formylation of furan is done by Gattermann reaction

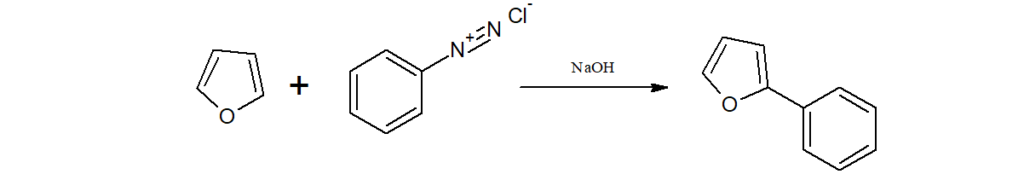
h)When an alkaline solution of furan is treated with benzenediazonium salts, 2-aryl furan is obtained.
i)When furan is treated with n-buytllithium, 2-lithiumfuran is obtained. Which when hydrolysed, furoic acid is formed.

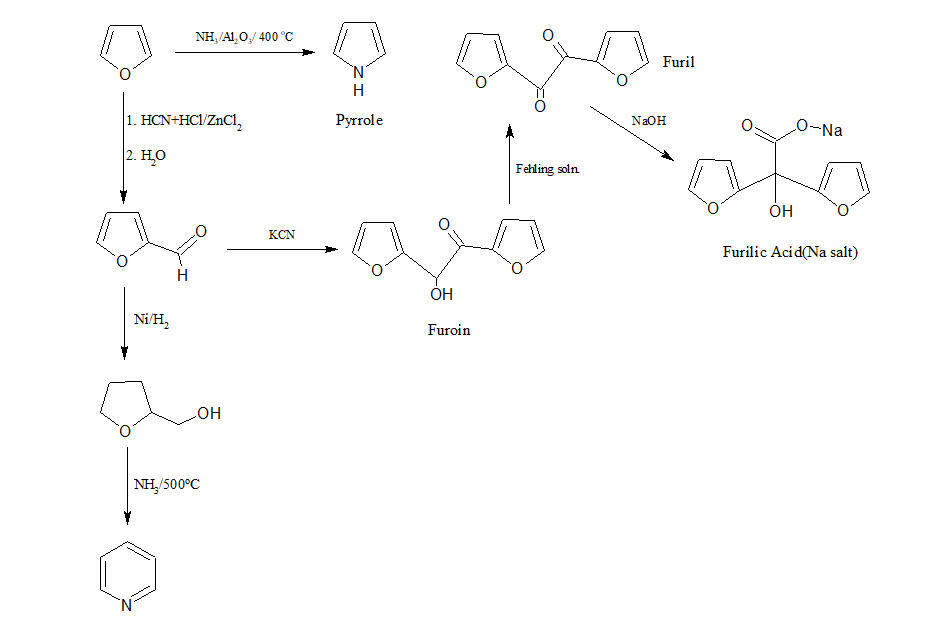
j)Conversion of furan to pyrrole, pyridine,Furoin, Furilic acid, furil.