The Ganem oxidation is a chemical reaction named after American chemist Dr. Bruce Ganem. It involves the oxidation of primary alcohols to carboxylic acids using pyridinium chlorochromate (PCC) as the oxidizing agent. The reaction typically takes place in dichloromethane or another suitable organic solvent at room temperature.
The general reaction scheme for the Ganem oxidation of a primary alcohol RCH2OH can be represented as follows:
RCH2OH+[CrO3Cl]−+H+→RCOOH+CrO3+HCl
In this reaction, pyridinium chlorochromate ([CrO3Cl]–) acts as the oxidizing agent. The primary alcohol is oxidized to a carboxylic acid, with the chromium compound being reduced in the process.
The Ganem oxidation is often preferred over other methods for oxidizing primary alcohols to carboxylic acids because it tends to provide high yields of the desired product and avoids overoxidation to the corresponding aldehyde or further oxidation to the carboxylic acid.
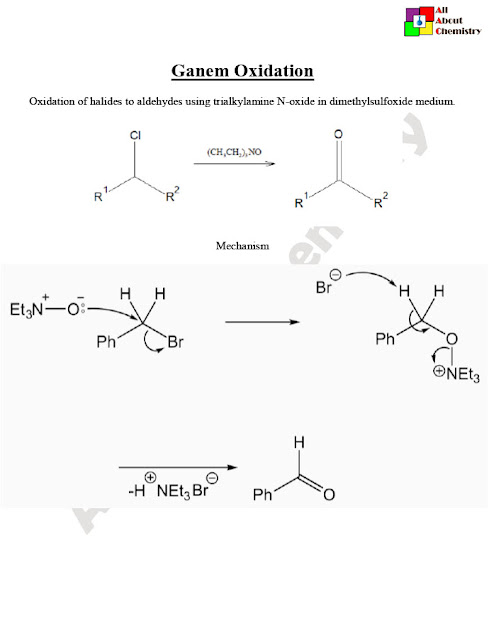
The mechanism of the Ganem oxidation involves several steps and intermediates. Here is a simplified version of the proposed mechanism:
- Formation of Chromyl Chloride (CrO2Cl2): Initially, pyridinium chlorochromate (PCC) reacts with a strong acid (usually HCl) to form chromyl chloride, which is the active oxidizing species in the reaction.
((C5H6N)[CrO3Cl]+HCl→(C5H6N)[CrO2Cl2]+H2O
- Coordination of Alcohol: The primary alcohol coordinates with the chromyl chloride complex.
RCH2OH+(C5H6N)[CrO2Cl2]→RCH2O−CrO2Cl2−HC5H6N
- Oxidation: A proton transfer occurs from the alcohol to the chromium atom, leading to the formation of a chromium ester intermediate. Simultaneously, the chromium atom undergoes a redox reaction, leading to the formation of a chromium(V) species.
RCH2O−CrO2Cl2−HC5H6N = RCH2O+−CrO2Cl2−Cl−HC5H6N
Cr(VI)→Cr(V)
- Water Elimination: The water molecule is eliminated from the chromium ester intermediate, resulting in the formation of a carbonyl compound coordinated with chromium.
RCH2O+−CrO2Cl2−Cl−HC5H6N→R−CrO2Cl2−Cl−HC5H6N+H2O
- Chromium(V) Reoxidation: Another molecule of chromyl chloride oxidizes the chromium(III) species back to chromium(V) and regenerates the active oxidizing species.
R−CrO2Cl2−Cl−HC5H6N+(C5H6N)[CrO3Cl]→RCOO−−CrO3Cl2−Cl−HC5H6N
- Protonation and Rearrangement: The coordinated carboxylate ion is protonated to yield the carboxylic acid product, while the chromium species undergoes rearrangement to form a more stable complex.
RCOO−−CrO3Cl2−Cl−HC5H6N = RCOOH+CrO3Cl+HC5H6N
Overall, the Ganem oxidation involves the coordination of the alcohol with the oxidizing agent, followed by oxidation, water elimination, reoxidation of chromium, and finally, protonation and rearrangement to yield the carboxylic acid product.
The Ganem oxidation, which involves the use of pyridinium chlorochromate (PCC) to oxidize primary alcohols to carboxylic acids, finds numerous applications in organic synthesis. Here are some of its key applications:
- Functional Group Transformations: The Ganem oxidation is widely used for the conversion of primary alcohols to carboxylic acids. This transformation is valuable in organic synthesis for introducing or modifying functional groups in various molecules.
- Natural Product Synthesis: Carboxylic acids are prevalent functional groups in many natural products. The Ganem oxidation provides a convenient method for the synthesis of carboxylic acid-containing natural products or their derivatives.
- Medicinal Chemistry: Carboxylic acids are frequently found in pharmaceutical compounds, serving as key pharmacophores or functional handles for further derivatization. The Ganem oxidation is employed in the synthesis of pharmaceutical intermediates and active pharmaceutical ingredients (APIs).
- Total Synthesis of Complex Molecules: The Ganem oxidation plays a crucial role in the total synthesis of complex molecules, such as natural products and biologically active compounds. It allows chemists to access key intermediates or target molecules by functionalizing alcohol groups present in the precursor molecules.
- Steroid Chemistry: Carboxylic acid functionalities are important in steroid chemistry, where they are often introduced through oxidation of the corresponding alcohols. The Ganem oxidation is utilized in the synthesis of steroid derivatives and analogs.
- Peptide Chemistry: Carboxylic acids are essential components in peptide synthesis, particularly for the synthesis of C-terminal amino acids. The Ganem oxidation can be employed in the preparation of peptide precursors or peptide fragments.
- Polymer Chemistry: Carboxylic acid-containing monomers are important building blocks in polymer chemistry. The Ganem oxidation can be used to introduce carboxylic acid groups into monomers, facilitating their polymerization and leading to the synthesis of functional polymers with desired properties.
Overall, the Ganem oxidation is a versatile tool in organic synthesis, offering a reliable method for the conversion of primary alcohols to carboxylic acids, which are valuable intermediates in various chemical processes and industries.















