The Gilman–Cason ketone synthesis is a method for the preparation of ketones from acid chlorides and organocopper reagents. It was developed independently by Henry Gilman and Joseph Cason in the 1950s. The reaction involves the addition of an organocopper reagent (such as a lithium dialkylcuprate or a Grignard reagent) to an acid chloride followed by hydrolysis.
Overall, the Gilman–Cason ketone synthesis provides a versatile and efficient method for the preparation of ketones from acid chlorides and organocopper reagents. It is widely used in organic synthesis for the construction of complex molecular structures.
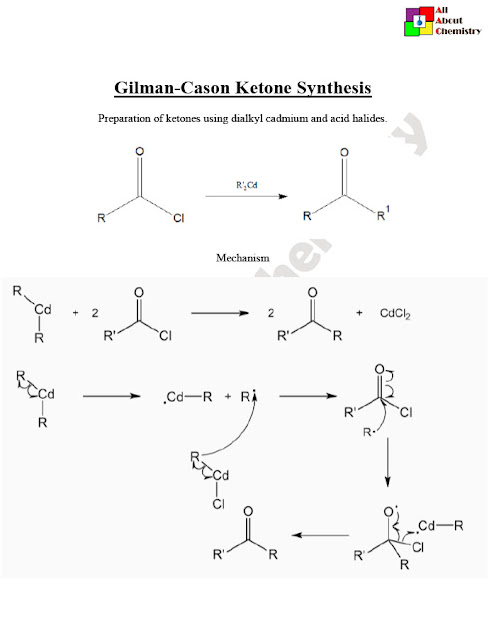
The Gilman–Cason ketone synthesis involves the addition of an organocopper reagent to an acid chloride followed by hydrolysis to form a ketone. Here’s a simplified mechanism for the reaction:
- Formation of the Organocopper Reagent: R-Li+CuX→R-CuLi+LiXR-Li+CuX→R-CuLi+LiX
- Addition of the Organocopper Reagent to the Acid Chloride: R-CuLi+R’COCl→R’R’C(OCuLi)RR-CuLi+R’COCl→R’R’C(OCuLi)R
- Hydrolysis: R’R’C(OCuLi)R+H2O→R’R’C(=O)R+CuOH+LiOHR’R’C(OCuLi)R+H2O→R’R’C(=O)R+CuOH+LiOH
In the first step, an organolithium compound (R-Li) reacts with copper halide (CuX) to form an organocopper reagent (R-CuLi) and lithium halide (LiX). This organocopper reagent is then added to the acid chloride (R’COCl) to form an alkyl or aryl ketone cuprate (R’R’C(OCuLi)R). Finally, hydrolysis of the ketone cuprate with water (H2O) yields the desired ketone (R’R’C(=O)R), along with copper hydroxide (CuOH) and lithium hydroxide (LiOH).
This mechanism outlines the key steps involved in the Gilman–Cason ketone synthesis. It’s worth noting that the actual reaction conditions and intermediates may vary depending on the specific organocopper reagent and reaction conditions used.
The Gilman-Cason Ketone Synthesis is a valuable tool in organic synthesis, offering a straightforward and efficient method for the preparation of ketones. Its applications are diverse and encompass various areas of organic chemistry. Here are some notable applications:
- Total Synthesis of Natural Products: Organic chemists frequently use the Gilman-Cason Ketone Synthesis in the total synthesis of complex natural products. By efficiently introducing ketone functionalities into target molecules, this method facilitates the construction of intricate molecular frameworks found in natural products.
- Medicinal Chemistry: Ketones are prevalent structural motifs in many pharmaceutical compounds. The Gilman-Cason Ketone Synthesis enables the introduction of ketone groups at specific positions in drug candidates, allowing medicinal chemists to modify pharmacological properties, improve potency, or explore structure-activity relationships.
- Functional Group Interconversion: The synthesis provides a means for converting acid chlorides into ketones, allowing for the interconversion of functional groups in organic molecules. This versatility is particularly useful in retrosynthetic analysis and the strategic design of synthetic routes.
- Organometallic Chemistry: The Gilman-Cason Ketone Synthesis involves the use of organocopper reagents, highlighting applications in organometallic chemistry. Organocopper compounds are versatile nucleophiles that participate in various synthetic transformations beyond ketone synthesis, including cross-coupling reactions and asymmetric synthesis.
- Method Development: Researchers continue to explore and refine the Gilman-Cason Ketone Synthesis, developing novel variants and improving reaction conditions. These efforts contribute to expanding the synthetic toolbox available to chemists and advancing the state of the art in organic synthesis.
- Industrial Synthesis: While many applications are in the realm of academic research, the Gilman-Cason Ketone Synthesis can also find practical use in industrial settings for the large-scale synthesis of ketone-containing compounds used in pharmaceuticals, agrochemicals, flavors, fragrances, and other fine chemicals.
Overall, the Gilman-Cason Ketone Synthesis holds significant importance in synthetic organic chemistry, offering a reliable and versatile method for the preparation of ketones, which are fundamental building blocks in organic synthesis. Its broad applicability underscores its enduring relevance in chemical research and development.








