The Hoffmann isonitrile synthesis is a chemical reaction named after its discoverer, August Wilhelm von Hofmann. It is a method for the preparation of isocyanides (isonitriles) from primary amines. The reaction involves treating a primary amine with chloroform (trichloromethane) and a strong base, typically a metal hydroxide like potassium hydroxide (KOH), in an alcoholic solvent such as ethanol or methanol.
The general reaction scheme is as follows:
RNH2+CHCl3+KOH→RNC+KCl+H2O
In this reaction:
- RNH₂ represents the primary amine.
- CHCl₃ is chloroform.
- KOH is potassium hydroxide.
- RNC represents the isocyanide product.
- KCl is potassium chloride.
- H₂O is water.
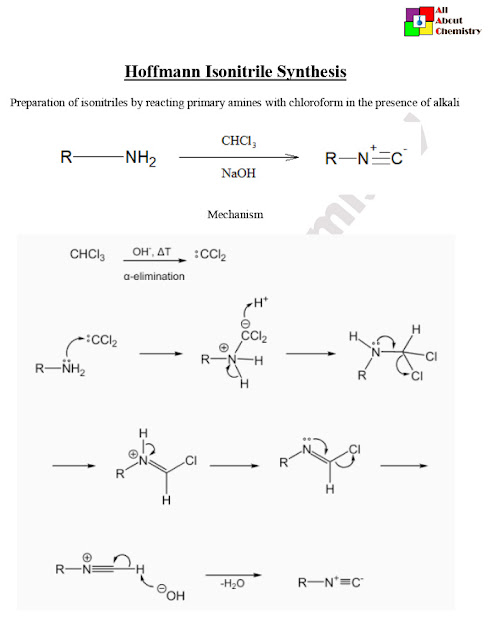
The mechanism of the Hoffmann isonitrile synthesis involves several steps. Here, I’ll outline the key steps involved in the reaction:
- Formation of Dichlorocarbene Intermediate: The reaction begins with the deprotonation of chloroform (CHCl₃) by the strong base (e.g., KOH) to form the trichloromethide ion (CHCl3−). The trichloromethide ion then reacts with the primary amine (RNH₂) to form an intermediate dichlorocarbene:CHCl3+KOH→CHCl2−+KCl+H2O CHCl2−+RNH2→RNH(Cl)+KCl+H2OHere, RNH(Cl) represents the chloroamine intermediate.
- Elimination of Hydrogen Chloride (HCl): The chloroamine intermediate undergoes an elimination reaction where a molecule of hydrogen chloride (HCl) is removed, leading to the formation of an isocyanide intermediate:RNH(Cl)→RN=C+HCl This step involves the abstraction of a proton from the nitrogen atom by the base, leading to the expulsion of HCl and formation of the isocyanide.
- Protonation of Isocyanide Intermediate: Finally, the isocyanide intermediate is protonated by the solvent or any available protons, resulting in the formation of the isocyanide product: RN=C+H2O→RN=C+H3O+This step ensures the stabilization of the isocyanide product.
Overall, the Hoffmann isonitrile synthesis involves the generation of a reactive dichlorocarbene intermediate from chloroform, followed by its reaction with a primary amine to form a chloroamine intermediate, which subsequently eliminates HCl to yield the desired isocyanide product.
The Hoffmann isonitrile synthesis has several applications in organic synthesis due to its ability to efficiently generate isocyanides from readily available starting materials. Some of the key applications include:
- Synthesis of Heterocycles: Isocyanides are versatile intermediates in the synthesis of various heterocycles, such as imidazoles, oxazoles, and thiazoles. These heterocyclic compounds are widely found in natural products and pharmaceuticals, making the Hoffmann isonitrile synthesis a valuable tool in medicinal chemistry and drug discovery.
- Multicomponent Reactions (MCRs): Isocyanides participate in numerous multicomponent reactions, where they serve as key building blocks for the construction of complex molecular scaffolds in a single step. The Hofmann isonitrile synthesis provides a convenient route to access these isocyanide building blocks, enabling the development of diverse MCRs for the synthesis of structurally diverse compounds.
- Transition Metal Catalysis: Isocyanides are ligands commonly employed in transition metal-catalyzed reactions. They can coordinate to transition metal centers and participate in various catalytic transformations, including carbon-carbon bond formation, cycloaddition reactions, and cross-coupling reactions. The availability of isocyanides through the Hoffmann isonitrile synthesis facilitates the exploration of new transition metal-catalyzed processes.
- Functional Group Transformations: Isocyanides undergo a wide range of functional group transformations, such as nucleophilic addition reactions, cycloadditions, and rearrangements. These transformations enable the synthesis of diverse functionalized molecules with valuable properties. The Hoffmann isonitrile synthesis provides a straightforward route to introduce the isocyanide functionality into organic molecules, allowing for subsequent functionalization.
- Chemical Biology: Isocyanides have also found applications in chemical biology, particularly in the development of probes for biological studies and imaging techniques. Isocyanide-based molecules can selectively target certain biological targets and be used as molecular probes to investigate biological processes. The Hoffmann isonitrile synthesis enables the preparation of isocyanide-based probes with tailored properties for specific biological applications.
Overall, the Hoffmann isonitrile synthesis plays a crucial role in organic synthesis and offers versatile opportunities for the preparation of diverse compounds with potential applications in medicinal chemistry, materials science, and chemical biology.










