Hydrogen-Occurrence-Isotopes
1. Introduction:
Alchemist Paracelsus, in the early 1500s noted that when iron filings were added to sulphuric acid colourless bubbles comes out. The same observation was made by Robert Boyle in 1671. In 1766 Henry Cavendish collected the bubbles and showed that when the gas burns it forms water. The gas was given its name hydro-gen, meaning water-former, by French chemist, Antoine Lavoisier.
Hydrogen is the first element of the periodic table. It is the lightest gas.
2. Position of hydrogen in the periodic table:
Although hydrogen occupies Group-1 in the periodic table, still it’s position in the periodic table is controversial as it shows resemblance with both alkali metals of group-1 and halogens of group-17. Thus hydrogen is called a rogue element.
a. Resemblance of hydrogen with alkali metals:
i) Electronic configuration:
Hydrogen contains a single electron in the valence shell like alkali metals.
| H | 1s1 |
| Li | 1s22s1 |
| Na | 1s22s22p63s1 |
| K | 1s22s22p63s23p64s1 |
ii) Electropositive nature:
Hydrogen like alkali metals can lose the single valence electron and form hydrogen ion, i.e, H+(proton).
H – e → H+
Na – e → Na+
Li – e → Li+
K – e → K+
iii) Oxidation number:
Like alkali metals, hydrogen shows only one oxidation state of +1 in its compounds.
iv) Reducing nature:
Like alkali metals, hydrogen acts as a strong reducing agent.
Fe3O4 + 4H2 3Fe + 4H2O
B2O3+6K 2B + 3K2O
v) Reaction with non-metals:
Hydrogen, like alkali metals can combine with non-metals .
| Non-metal | Compound of hydrogen | Compound of alkali metal |
| O2 | H2O | Na2O |
| Cl2 | HCl | NaCl |
| S | H2S | Na2S |
vi) Liberation at the cathode:
When an aqueous solution of HCl is electrolyzed, H2 is liberated at the cathode in the same way as alkali metals are liberated at the cathode during the electrolysis of their fused halides.
At cathode At anode
2HCl(aq) → H2(g) + Cl2(g)
2NaCl(l) → 2Na(l) + Cl2(g)
b. Resemblance of hydrogen with halogens:
i) Atomicity:
Hydrogen is diatomic like halogens.
H2 , F2 , Cl2 , Br2 , I2
ii) Non-metallic nature:
Like halogens , hydrogen also acts like a non-metal.
iii) Combination with metals:
Hydrogen can combine with metals like halogens.
H2 + 2Na → 2NaH
2Na + Cl2 → 2NaCl
iv) Electronegative nature:
Hydrogen can also gain electron to form anions like halogens.
H + e →H–
Cl + e →Cl–
v) Oxidation number:
Like halogens, hydrogen also shows -1 oxidation state in hydrides.
vi) Ionisation potential:
The ionisation potential of halogen and hydrogen are quite comparable.
vii) Liberation at anode:
When metal hydrides are electrolysed in molten state, hydrogen is liberated at the anode. Similarly when any metal halide is electrolysed in molten state, halogen gas is liberated at the anode.
viii) Physical state:
Hydrogen is also a gas like fluorine and chlorine.
ix) Electronic configuration:
Like halogen, hydrogen also requires a single electron to attain stable electronic configuration.
x) Formation of stable covalent compound:
Like halogen, hydrogen can also form stable compounds with non-metals.
| Non-metal | Hydrogen compound | Halogen compound |
| Carbon | CH4 | CCl4 |
| Nitrogen | NH3 | NCl3 |
| Silicon | SiH4 | SiCl4 |
| Sulphur | H2S | SCl6 |
| Phosphorous | PH3 | PCl3 |
Difference between hydrogen and alkali metals/halogen
- Hydrogen form neutral oxide. While alkali metals form basic oxide and halogens form acidic oxide.
- Hydrogen is not solid like alkali metals.
- Hydrogen is not so active like alkali metals. It remains in free state in elemental form, which alkali metals cannot.
- Hydrogen does not contain lone pair electrons like halogens.
- Hydrogen can combines with non-metallic ions to form acids and organic molecules. Halogens can form negatively-charged ions that react with metallic, ions to form salts.
3. Occurrence:
Hydrogen is the most abundant element in the universe (7.5 x 105 ppm). It is also found in Sun (7.5 x 105 ppm), earth’s crust (1500 ppm), sea water (107800 ppm), meteorite (24000 ppm) and in our body (1 x 108 ppb).Stars contain unlimited supply of hydrogen. Giant planets like Jupiter and Saturn mostly contain hydrogen.Hydrogen occurs in traces in volcanic gases.Meteorites which are composed of iron , nickel and cobalt also contain hydrogen.It occurs chiefly in combined states as methane, ethane, ethene, ethyne, benzene, carbohydrates, hydracids etc.
The extreme high temperature of the sun is due to nuclear fusion of hydrogen atoms liberating a large amount of energy.
4 1H1 → 2He4 + -1e0 + Energy
| Abundance of hydrogen in | Position |
| Universe | 1st |
| Earth crust | 10th |
| Solar system | 1st |
| Sun | 1st |
| Earth’s mantle | 14th |
| Bulk Earth | 16th |
| Earth’s atmosphere | 10th |
| Ocean water | 2nd |
| Human body | 3rd |
| Human muscles | 3rd |
| Human bones | 4th |
| River water | 2nd |
4. Isotopes of hydrogen:
Hydrogen has three stable isotopes protium(1H1), deuterium(1H2) and tritium(1H3). Beside these isotopes, 1H4, 1H5,1H6,1H7 are also possible. These four isotopes are radioactive with very little half-life.
| Isotope | Half Life |
| 1H4 | 139 yoctoseconds |
| 1H5 | 910 yoctoseconds |
| 1H6 | 290 yoctoseconds |
| 1H7 | 23 yoctoseconds |
| Properties | Protium(1H1) | Deuterium(1H2) | Tritium(1H3) |
| Symbol | H | D | T |
| Number of neutrons | 0 | 1 | 2 |
| Relative abundance | 99.98% | 0.0026-0.0184 | 10-15 |
| Nuclear spin | +1/2 | +1 | +1/2 |
| Relative mass | 1.00782503207 | 2.0141017778 | 3.0160492777 |
| Radioactive nature | Non-radioactive | Non-radioactive | Radioactive. emitter |
| Half-life | – | – | 12.33years |
| Melting point(0C) | -259 | -254.3 | -252.4 |
| Boiling point(0C) | -252.6 | -249.3 | -248.0 |
| Latent heat of vaporisation(kJ/mol) | 0.904 | 1.226 | 1.393 |
| Latent heat of fusion(kJ/mol) | 0.117 | 0.197 | 0.250 |
| Density(g/L) | 0.09 | 0.18 | 0.27 |
| Critical temperature(0C) | -239.8 | -234.6 | -232.4 |
| Nuclear magnetic moment (μ/μN) | 2.7928456 | 0.8574376 | 2.978960 |
| Binding energy | 0 | 2224.52± 0.20 keV | 8,481.821± 0.004 keV |
| Uses | i. Protium is used in hydrogenation of nitrogen, carbon monoxide , coal and unsaturated or liquid fats . ii. Protium is used to prepare pure tungsten . iii.It is used in the production of high temperature by suggests of oxyhydrogen flame for welding purposes. iv. Protium is used in filling balloons and dirigibles. | i. Deuterium is applied as a tracer, in different physical, chemical and biological reaction. ii. Heavy water (D2O) is used in nuclear fission reactors to slow down the fission process. iii. Deuterium also act as a potential fuel for commercial nuclear fusion. | i. Tritium is used to produce betalights, which are now used in watches. ii. It is used to enhance the efficiency and yield of fission bombs. iii. It is used in making different thermonuclear device. |
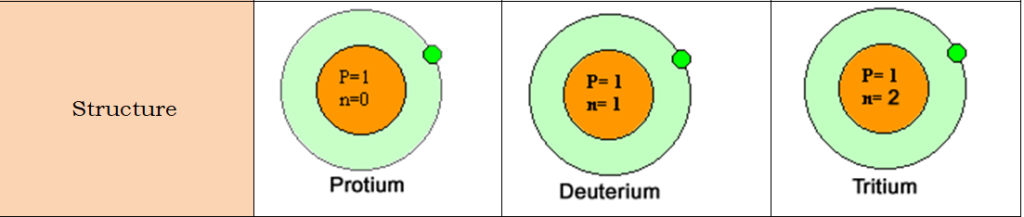
Due to the difference in the atomic masses of the isotopes, they possess different physical properties and reaction rates but the same chemical properties. This is called the isotope effect. Protium reacts 13.4 times faster than deuterium with chlorine gas.
As You complete this portion don’t forget to complete the following Review Questions.
Review Questions
- Why hydrogen is called ‘Rogue element’?
- Name the isotopes of hydrogen.
- Which is the most abundant element in-universe?
- Which isotope of hydrogen is used to extract pure tungsten?
- Name the isotope of hydrogen used to prepare heavy water.
- Which is the heaviest isotope of hydrogen?
- What is the isotope effect?
- Name the isotope of hydrogen which does not contain neutron.
- Why is the abundance of hydrogen so less in the atmosphere?
- Why the reaction rates with different isotopes of hydrogen are different?
You may also Read:
https://www.britannica.com/science/hydrogen
https://www.rsc.org/periodic-table/element/1/hydrogen
https://pubchem.ncbi.nlm.nih.gov/compound/Hydrogen














