Test for lead ion (Pb2+ ion)
[ Sample : lead nitrate solution or lead acetate solution]
| Reagent | Precipitation formed | Colour of ppt |
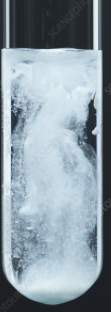
| H2SO4 or Na2SO4 | PbSO4 | White ppt insoluble in dil acid. |
| HCl | PbCl2 | White ppt soluble in hot water |
| H2S | PbS | Black ppt soluble in conc. HNO3 |
| K2CrO4 | PbCrO4 | Yellow ppt insoluble in dil. Acetic acid |
| KI | PbI2 | Yellow ppt formed which dissolves in boiling water to a colourless solution, which deposits as brilliant yellow crystals upon cooling. |
| NaOH | Pb(OH)2 | Chalky white ppt , dissolves in excess NaOH solution. |
| NH4OH | Pb(OH)2 | Chalky white ppt , dissolves in excess NH4OH solution. |





Test for Silver ion (Ag+)
[ Sample: silver nitrate solution]
| Reagent | Precipitation formed | Colour of ppt |
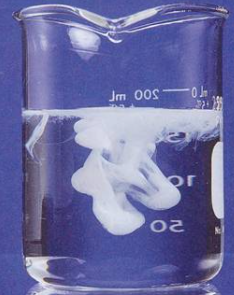
| NaCl or HCl | AgCl | Curdy white ppt, soluble in ammonium hydroxide but reappears on adding HNO3. |
| KI | AgI | Yellow ppt insoluble in ammonium hydroxide. |

Test for Copper ion (Cu2+)
[ Sample : copper sulphate solution]
| Reagent | Precipitation formed | Colour of ppt |
| NaOH | Cu(OH)2 | Pale blue ppt, insoluble in excess NaOH solution. |
| NH4OH | Cu(OH)2 | Pale blue ppt, soluble in excess NH4OH solution to produce an inky blue solution. |
| K4[Fe(CN)6] | Cu2[FeCN)6] | Chocolate brown ppt. |
| H2S | CuS | Black ppt. |
| Cu+ ion | Cu2+ ion |
| When NH4OH solution is added a pale blue ppt is formed which dissolves in excess to form an inky blue solution. | When NH4OH solution is added a pale yellow ppt is formed which dissolves in excess to form a colourless solution. |
| Cu(OH)2 when heated produce a black residue of CuO. | Cu2(OH)2 when heated produce a red residue of Cu2O. |



Test for Ferrous ion (Fe2+)
[ Sample : ferrous sulphate solution]
| Reagent | Precipitation formed | Colour of ppt |
| NaOH | Fe(OH)2 | Dirty green ppt, insoluble in excess NaOH solution. |
| NH4OH | Fe(OH)2 | Dirty green ppt, insoluble in excess NH4OH solution. |
| K4[Fe(CN)6] | K2Fe[Fe(CN)6] | Bluish white ppt formed which turns deep blue due to aerial oxidation. |
| K3[Fe(CN)6] | KFe[Fe(CN)6] | Deep blue ppt.(Turnbull’s blue) |

Test for Ferric ion (Fe3)
[ Sample : ferric chloride solution]
| Reagent | Precipitation formed | Colour of ppt |
| NaOH | Fe(OH)3 | Reddish brown ppt, insoluble in excess NaOH solution. |
| NH4OH | Fe(OH)3 | Reddish brown ppt, insoluble in excess NH4OH solution. |
| K4[Fe(CN)6] | KFe[Fe(CN)6] | Deep blue ppt.(Prussian blue) |
| K3[Fe(CN)6] | Fe[Fe(CN)6] | Brown colouration. |
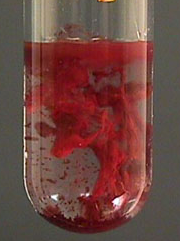
| NH4SCN | Fe(SCN)3 | Blood red coluration |

Ferric hydroxide


Test for Aluminium ion (Al3+)
[ Sample : aluminium nitrate solution]
| Reagent | Precipitation formed | Colour of ppt |
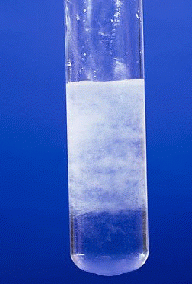
| NaOH | Al(OH)3 | Gelatinious white ppt formed which dissolves in excess NaOH solution. |
| NH4OH | Al(OH)3 | Gelatinious white ppt formed which is insoluble in excess NH4OH solution. |
Test for Zinc ion (Zn2+)
[ Sample : zinc nitrate solution]
| Reagent | Precipitation formed | Colour of ppt |
| NaOH | Zn(OH)2 | Gelatinious white ppt formed which dissolves in excess NaOH solution. |
| NH4OH | Zn(OH)2 | Gelatinious white ppt formed which dissolves in excess NH4OH solution. |
| K4[Fe(CN)6] | K2Zn3[Fe(CN)6]2 | Bluish white ppt formed. |
| (NH4)2[Hg(SCN)4] + CuSO4 | Zn[Hg(SCN)4]+ Cu[Hg(SCN)4] | Violet ppt formed. |

zinc hydroxide

ferrocyanide
Test for Calcium ion (Ca2+)
[ Sample solution contain zinc nitrate solution]
| Reagent | Precipitation formed | Colour of ppt |
| NaOH | Ca(OH)2 | White ppt formed which is insoluble in excess NaOH solution. |
| Oxalic acid | CaC2O4 | Crystalline white ppt |


Test for Anions:
| Experiment | Observation | Inference |
| Sample solution is treated with lead nitrate solution | Black ppt of lead sulphide produced. | S2- ion |
| Sample solution is treated with sodium nitroprusside solution. | Violet colouration | S2- ion |
| Sample solution is treated with Silver nitrate solution. | Curdy white ppt of silver chloride soluble in NH4OH solution but reappears on adding HNO3. | Cl– ion |
| Sample solution is treated with Silver nitrate solution. | Pale yellow silver bromide ppt produced which dissolves in excess NH4OH | Br– ion |
| Sample solution is treated with Silver nitrate solution. | Yellow ppt of silver iodide produced which is insoluble in NH4OH | I– ion |
| Sample solution is treated with Silver nitrate solution. | White ppt of silver thiosulphate produced which changes to yellow to orange to red to brown to black. | S2O32- ion |
| Sample solution is treated with BaCl2 solution. | White ppt barium sulphate produced which is insoluble in dil. HCl. | SO42‑ ion |
| Sample solution is treated with BaCl2 solution. | White ppt of barium sulphite produced which is soluble in dil. HCl. | SO32- ion |
| Sample solution is treated with calcium chloride solution. | Crystalline white ppt of calcium oxalate formed. | C2O42- ion |
| Sample solution is treated with lead acetate solution | Yellow ppt lead chromate produced insoluble in acetic acid but soluble in dil HNO3. | CrO42- ion |
| Sample solution is treated with silver nitrate solution | Reddish brown ppt of silver chromate produced. | CrO42- ion |
| Sample solution is treated with ferric chloride solution | Deep red colouration which produce a reddish brown ppt on heating. | CH3COO– ion |

Test for different gases:
1.Test for Carbon dioxide gas
Carbon dioxide is a colorless and odourless gas which turns lime water milky but cannot change the orange colour of acidified potassium dichromate solution.

2.Test for Sulphur dioxide gas
Sulphur dioxide is a suffocating smelling colourless gas which turns lime water milky and turns acidified potassium dichromate solution green from orange.

3.Test for Chlorine gas
It is a greenish yellow gas with bleaching powder like smell which turns Starch KI paper Blue black.


4.Test for Ammonia gas
It is colourless pungent smelling gas. It turns nessler’s Reagent brown.

5.Test for Hydrogen sulphide gas
It is a colourless gas with a rotten egg smell. It turns lead nitrate solution black.

6.Test for Oxygen gas
It turns alkaline pyrogallol solution brown

7.Test for hydrogen
Pure hydrogen gas burns with a pale blue flame.

8.Test for Nitrogen dioxide
It is a reddish brown gas. It turns green acidified ferrous sulphate solution brown.

9.Test for water vapour
- It turns white anhydrous copper sulphate to blue ( copper sulphate pentahydrate).
- It also turns blue cobalt chloride paper pink.


Heating effects
1.Heating copper carbonate
When light green copper carbonate is heated, black copper oxide is produced along with carbon dioxide gas.


2. Heating zinc carbonate
When white zinc carbonate is heated, zinc oxide is produced which is yellow when hot and white when cold along with carbon dioxide gas.


3. Heating sodium carbonate decahydrate
When white crystalline solid is heated, a white anhydrous sodium carbonate is formed along with water vapour.


4. Heating copper sulphate pentahydrate
When blue crystalline copper sulphate pentahydrate is heated, white amorphous anhydrous copper sulphate is formed.
5. Heating zinc nitrate
When white zinc nitrate is heated, zinc oxide(which is yellow when hot and white when cold), nitrogen dioxide and oxygen is produced.

6. Heating copper nitrate
When blue copper nitrate is heated, black copper oxide, nitrogen dioxide and oxygen is produced.

7. Heating lead nitrate
When white lead nitrate is heated, yellow lead oxide, nitrogen dioxide and oxygen is produced.

8. Heating Iodine
When grey colour solid iodine is heated, violet vapour of iodine is produced.


9. Heating ammonium dichromate
Orange red ammonium dichromate when heated produce greenish grey chromic oxide, nirogen gas and water.



Test for Acids
1.Test for Hydrochloric Acid

i)When hydrochloric acid is added to colourless silver nitrate solution, curdy white ppt of silver chloride is produced which dissolves in ammonium hydroxide solution.
ii)When Hydrogen chloride gas comes in contact with a glass rod dipped in ammonium hydroxide solution, a dense white fumes of ammonium chloride is produced.

2. Test for Sulphuric acid

i) When colourless barium chloride solution reacts with sulphuric acid, a white ppt of barium sulphate is produced which is insoluble in dilute HCl.

ii) When lead acetate or lead nitrate solution is added to sulphuric acid, a white ppt of lead sulphate is produced.
3. Test for Nitric acid

Nitric acid is treated with freshly prepared ferrous sulphate. Then the test tube is cooled in running tap water. After that, conc.sulphuric acid is added along the side of the test tube. A prominent brown ring is formed at the junction of the two liquids.

Bibliography
https://en.wikipedia.org/wiki/Lead(II)_sulfate
https://www.sciencephoto.com/media/779949/view/lead-sulphate-precipitate-3-of-3
https://en.wikipedia.org/wiki/Lead(II)_chloride
https://www.sciencephoto.com/media/859974/view/lead-sulphide-precipitation-reaction
https://www.sciencephoto.com/media/860516/view/lead-sulphide-precipitate-3-of-3
https://www.sciencephoto.com/media/780526/view/lead-chromate-precipitate
https://www.flickr.com/photos/paigggeyy/5533819494
https://www.sciencephoto.com/media/780493/view/lead-ii-hydroxide-precipitate
https://www.sciencesource.com/archive/Silver-chloride-precipitate-SS2697807.html
https://www.sciencephoto.com/media/945459/view/silver-nitrate-test-for-iodides
https://www.sciencephoto.com/media/4345/view/copper-hydroxide-precipitate
https://www.sciencephoto.com/media/860524/view/copper-sulphide-precipitate-3-of-3
https://www.sciencephoto.com/media/992276/view/iron-ii-hydroxide-precipitate]
https://www.sciencephoto.com/media/706620/view/iron-iii-hydroxide-precipitate
https://www.youtube.com/watch?v=GwYDLH3uMIk
https://pixels.com/featured/aluminium-hydroxide-precipitate-andrew-lambert-photography.html
https://www.sciencephoto.com/media/706614/view/zinc-hydroxide-precipitate
https://pixels.com/featured/calcium-hydroxide-precipitate-andrew-lambert-photography.html
https://www.sciencephoto.com/media/4580/view/silver-chromate-precipitation
https://www.indiamart.com/proddetail/iron-acetate-or-ferric-acetate-solution-20252371333.html
https://www.bbc.co.uk/bitesize/guides/z6dtgwx/revision/1
https://assets.pearsonglobalschools.com/asset_mgr/current/201214/Sp_1_1_10.pdf
https://www.tradeindia.com/fp4311616/Liquid-Chlorine-Gas.html
http://www.sciencemadness.org/talk/viewthread.php?tid=68620
https://www.quora.com/Which-is-the-gas-that-turns-Nesslers-Reagent-pale-brown
https://www.youtube.com/watch?v=If9goM5wci4
https://www.sciencephoto.com/media/706788/view
https://www.alamy.com/stock-photo/copper-sulfate.html
https://www.slideshare.net/prkppt/2emulsion
https://www.ceramic-glazes.com/pigments-and-stains-copper-carbonate
https://www.indiamart.com/proddetail/cupric-oxide-black-2965497330.html
https://www.ec21.com/product-details/High-Quality-Zinc-Carbonate–9348640.html
https://www.indiamart.com/proddetail/heated-zinc-oxide-10515984812.html
https://www.imperialchemicals.in/sodium-carbonate-anhydrous.html
https://www.sciencephoto.com/media/9740/view/zinc-nitrate-crystals
https://onlinechina.shop/p-1600121566023
https://www.indiamart.com/proddetail/lead-nitrate-20343622188.html
https://www.britannica.com/science/iodine
https://www.sciencephoto.com/media/4109/view/iodine-crystals-and-vapour
https://www.amazon.in/BFC-AMMONIUM-DICHROMATE-LR-7789-09-5/dp/B072MWSB2N
https://www.indiamart.com/proddetail/green-chromium-oxide-2522130048.html
https://www.chemistryworld.com/podcasts/ammonium-dichromate/7406.article
https://www.indiamart.com/proddetail/cp-sulphuric-acid-10341965473.html
https://www.indiamart.com/proddetail/dilute-hydrochloric-acid-15050712648.html
https://www.indiamart.com/proddetail/liquid-fuming-nitric-acid-17467382730.html
https://en.wikipedia.org/wiki/Nitrate_test















