Alkenes are compound which possesses double bond and alkynes are compounds which possesses triple bond in it. Alkenes and alkynes are also called unsaturated compounds.
Rules for the nomenclature of alkenes and alkynes.
- Select the longest chain with maximum number of multiple bonds.
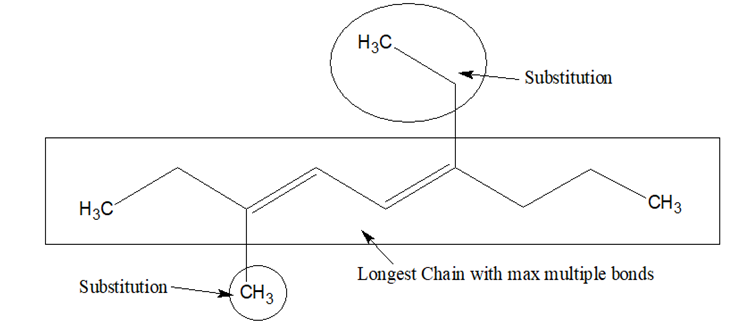
- If there is only one multiple bond, in the principal chain, the numbering of the chain is done in such a way that the carbon atom containing the multiple bond gets the lowest locant.
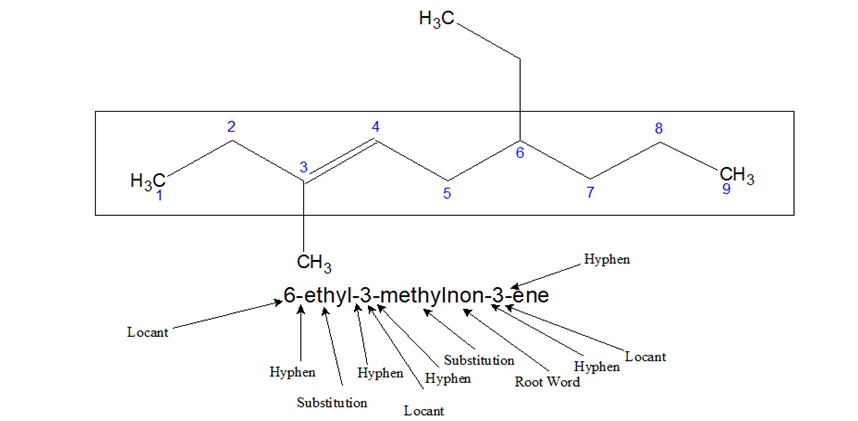
- When the name is written the following method is used-
Locant-Substitution-Root Word-locant-ene
For double bond, ‘ene’ is used and for triple bond, ‘yne’ is used.
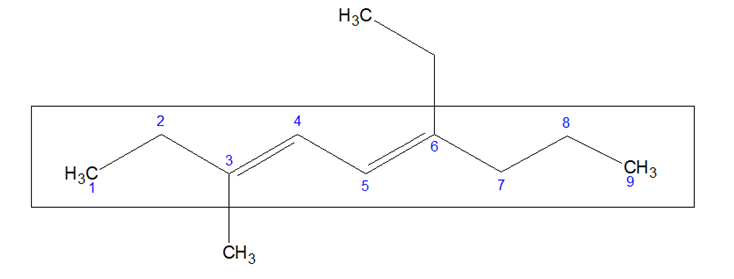
- If two or more multiple bonds are present, numbering is done in such a way that the summation of the locants of the multiple bonds is minimum.
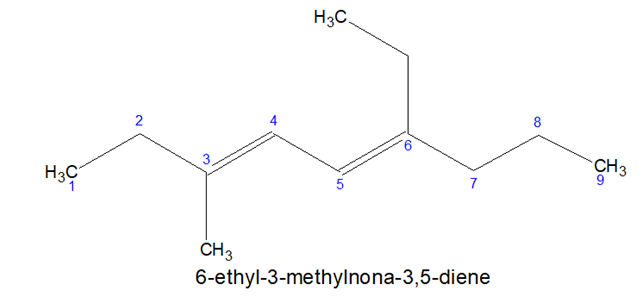
- If the suffix starts with a,i,o,u,y, then, vowel is not written immediately before it.
Note: Non-3-ene but not Nona-3-ene.
Again, Nona-3, 5-diene not Non-3, 5-diene
- If from both terminals, double and triple bonds are in the same locants, double bond is given more priority.

- Multiple bonds are given more priority than substitution.
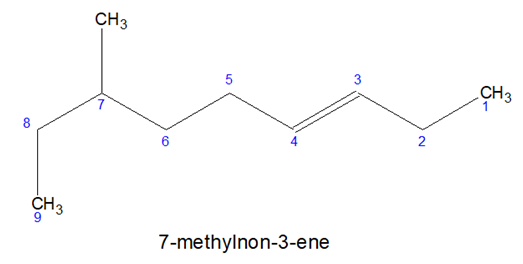
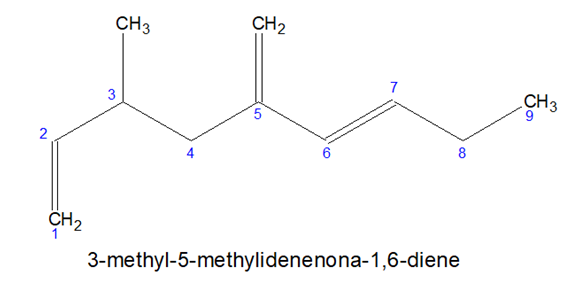
- Sometimes it is not possible to accommodate all the multiple bonds in the chosen parental chain, in such cases, some prefixes can be used-
CH2= Methylidene , CH3CH= Ethylidene

After completing the rules try out the Practice Set (CLICK)