Acid Chloride are the derivatives of carboxylic acids which have –COCl group in it.
Rules:
1.Start numbering from the carbon of –COCl.
2.For naming: alkane-e+ oyl chloride

In the above compound, five carbon is present.
Thus the name of the compound is- pentane-e + oyl chloride
3.If two -COCl group is present, always keep the -COCl on the two terminals irrespective of the length of the chain.
4.It is named as: Alkanedioyl dichloride

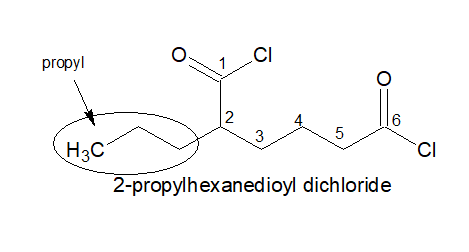
5.If three -COCl is present, the stem chain holding the -COCl group directly is considered as the parental chain.
6. It is named as : Alkane-x,y,z-tricarbonyl trichloride
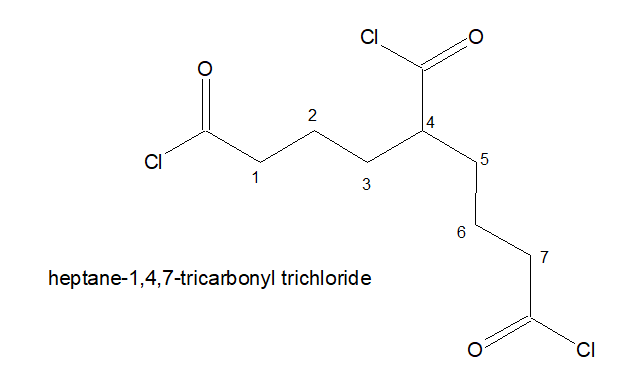
7.If three -COCl is present but of it is not directly attached to the stem chain. Then the longest chain holding two -COCl in the terminals are chosen as the parental chain. and the 3rd -COCl is named as : chloro oxo alky

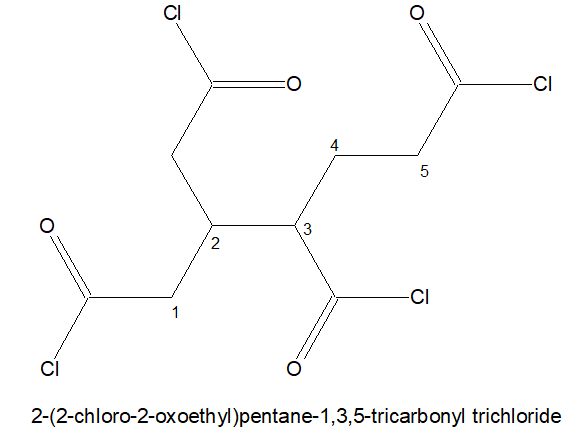
Try out the following:
Draw the structure of the following
- 4-(2-chloro-2-oxoethyl)-3-methylnonanedioyl dichloride
- 2,5-dichlorohex-3-enedioyl dichloride
- 4,5-bis(2-chloro-2-oxoethyl)octanedioyl dichloride
- 4-(2-chloro-2-oxoethyl)-6-(1-chloro-1-oxopropan-2-yl)nonanedioyl dichloride
- hepta-2,6-dien-4-yne-1,2,7-tricarbonyl trichloride
- 4-(4-chloro-4-oxobutan-2-yl)-5-(2-chloro-2-oxoethyl)octane-1,3,8-tricarbonyl trichloride
Answer:https://www.allaboutchemistry.net/practice-set-acid-chloride/















