Alkanes are saturated acyclic hydrocarbons having carbon-carbon single bond. Alkanes are also known as paraffins and are represented by CnH2n+2 . Don’t forget to complete the PRACTICE SET (CLICK)
Procedure of nomenclature:
1.First of all we need to remember the word roots. Depending upon the number of carbon in a carbon we use different word roots. Remember, during nomenclature, we are interested only in the carbon.
| No of Carbon | Word Root |
| 1 | Meth |
| 2 | Eth |
| 3 | Prop |
| 4 | But |
| 5 | Pent |
| 6 | Hex |
| 7 | Hept |
| 8 | Oct |
| 9 | Non |
| 10 | Dec |
| 11 | Undec |
| 12 | Dodec |
| 13 | Tridec |
| 14 | Tetradec |
| 15 | Pentadec |
| 16 | Hexadec |
| 17 | Heptadec |
| 18 | Octadec |
| 19 | Nonadec |
| 20 | Icos |
| 21 | Henicos |
| 22 | Docos |
| 23 | Tricos |
| 24 | Tetracos |
| 25 | Pentacos |
| 26 | Hexacos |
| 27 | Heptacos |
| 28 | Octacos |
| 29 | Nonacos |
| 30 | Triacont |
| 31 | Hentriacont |
| 32 | Dotriacont |
| 33 | Tritriacont |
| 40 | Tetracont |
| 50 | Pentacont |
| 60 | Hexacont |
| 100 | Hecta |
| 200 | Dicta |
| 1000 | Kilia |
| 2000 | Dilia |
2.Search for the longest chain with maximum number of substitution. The longest chain is also known as stem chain.
3.Substitutions are the alkyl groups which come out from the stem chain.
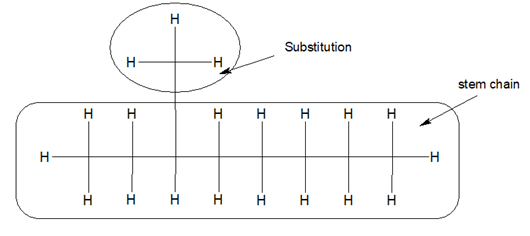
Substitutions are also named based on the word roots. In the example given above, we have total 8 carbon in the stem chain. Thus it is Oct. and the substitution carries a single carbon, thus it is meth.
4. As we are dealing with alkanes, we will add “ane” after the root word. For substitution, we will add “yl” after the root word.
So the above example will become, octane and methyl respectively.
5. Substitutions are written first followed by the name of the stem chain without any space in between.
So, the above example will methyloctane but not methyl octane.
Note: methyloctane will act as a single word.
6. If a molecule has two or more chains, the chain with highest number of substititions will be considered as the stem chain.
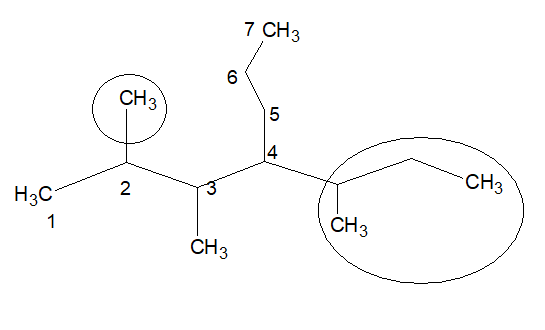
In this example, if the numbering is done in the way it is shown, we get two substitutions .{Don’t consider the nature of substitution is, just count the number of substitutions}
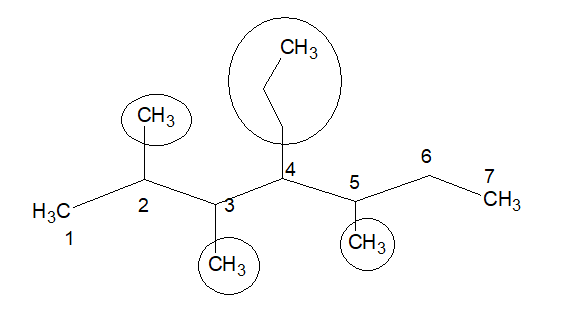
But if it is numbered in this way, we get 4 substitutions. So this is the correct stem chain.
7. Lowest set of locants rule:
Numbering should start from that end of the stem chain, where we get the minimum locant of the first substitution. Locant is the position of the substitution.
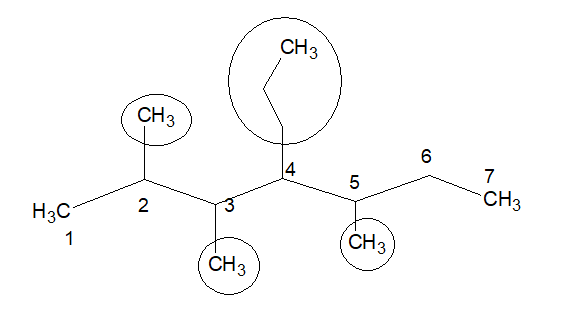
In this example, the locants are-2,3,4,5 (the points from which the substitutions are coming out). Here if we start numbering from the left corner, the first substitution comes out from locant-2.
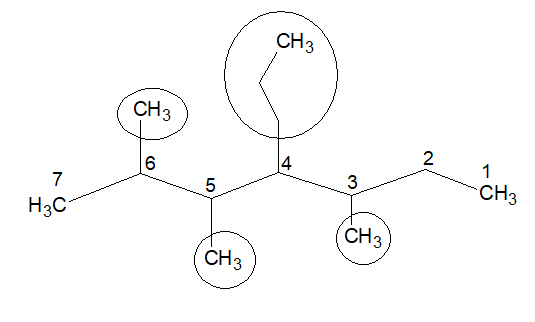
If we start numbering from the right end, the first substitution will come out from locant-3.
So, we will use the previous numbering.
8.If there are two substitutions at equal distance from both terminus of the chain, use the first alphabet of the name of the substitution, irrespective of the size of the substitutions.
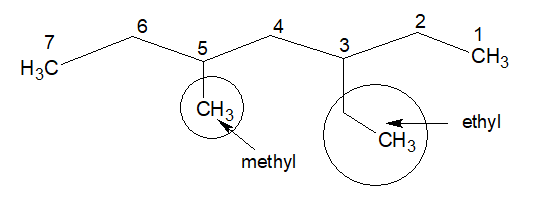
“E” of ethyl is given more priority than “m“of methyl in the above example.
9. Lowest Sum Rule:
If more than two substitutions are present, add the locants of all the substitutions. The lowest summation value will be taken into account. [Always remember- first give priority to LOWEST SET OF LOCANTS RULE then LOWEST SUM RULE]
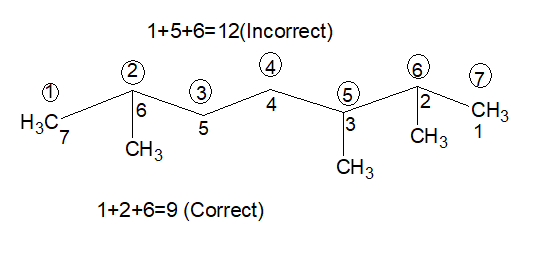
10.For more than one identical substituents, prefix is added before their name.
| Number | Numerical Prefix | Number | Numerical Prefix |
| 2 | Di | 7 | Hepta |
| 3 | Tri | 8 | Octa |
| 4 | Tetra | 9 | Nona |
| 5 | Penta | 10 | Deca |
| 6 | Hexa | 11 | Undeca |
Remember- Numerical prefixes are not counted when the alphabetic order is choose.
Suppose we have, trimethyl, “m” of methyl will be taken to decide the priority of the substitution.
11.In between two numbers, ‘Comma’ and in between a number and a word, ‘hyphen’ is given 12. So, the correct order of writing the name of an alkane is-
Locant hyphen substitution wordroot ane
or, Locant comma locant hyphen numeric prefix substitution wordroot ane
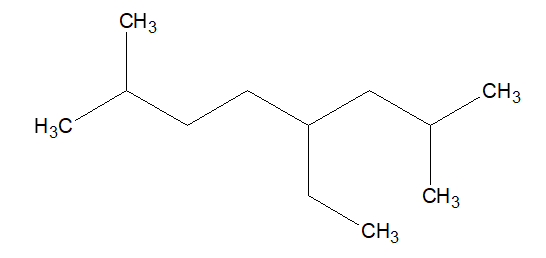
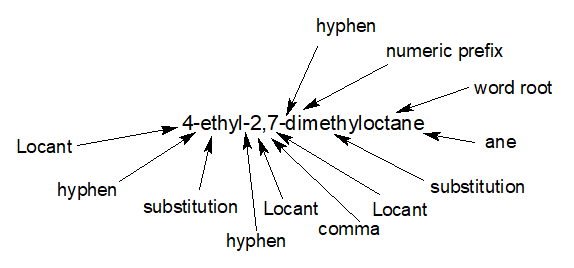
IUPAC Nomenclature of branch of branching
In case a substituent has branched chain, it is called complex chain.
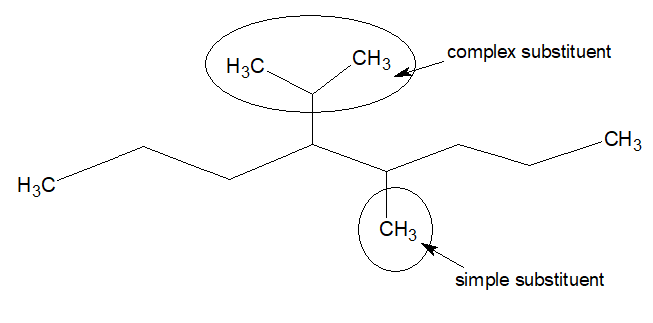
In such cases the following rules are used for naming the branch of the substituent.
FIRST METHOD
1.The first carbon of the longest chain coming out from the stem chain is numbered as 1.
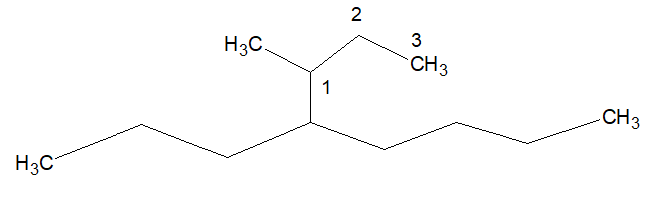
In the above example propyl is the longest chain coming out from the stem chain. Now from 1 position of that propyl group, a methy group is coming out.
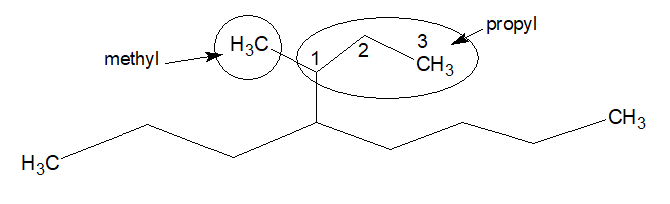
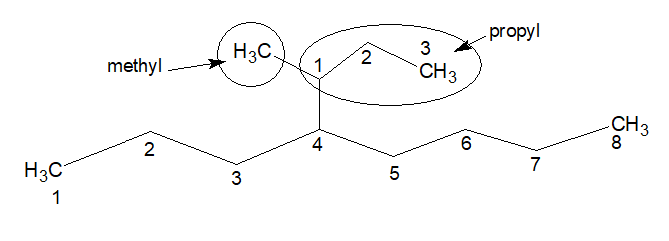
Name of the substitution is (1-methylpropyl) and the complex substitution is coming out from the locant 4, thus it forms, 4-(1-methylpropyl).
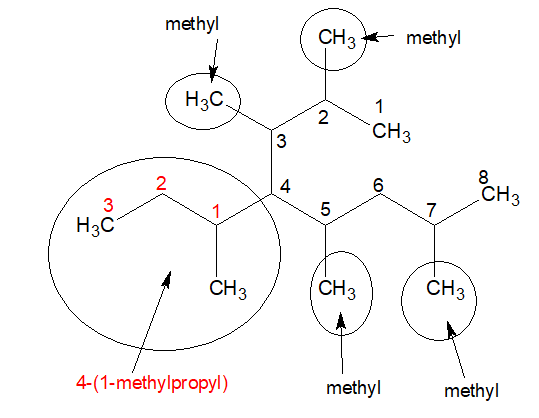
The name of the above compound the complex substituent is 4-(1-methylpropyl) and the simple substuituents are 2,3,5,7-tetramethyl.
2. While deciding the alphabetical order of various substituents, the name of the complex substituent is considered, to begin with, the first alphabet of its complex name. Here we will include “di”, ‘tri” etc as it forms a complete single substituent.
3.If identical complex substituents are present the following prefixes are added –
| 2 | bis |
| 3 | tris |
| 4 | tetra |
| 5 | penta |
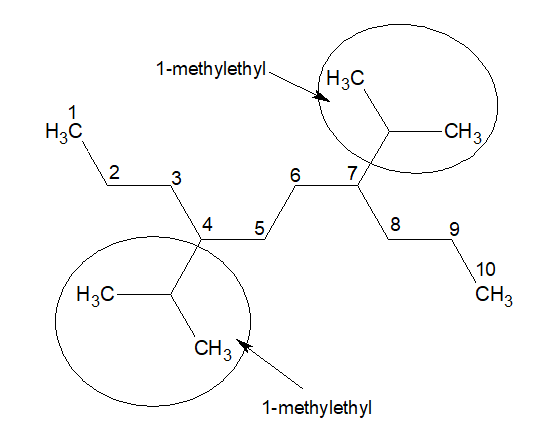
The name of the above compound is 4,7-Bis(1-methylpropyl)decane
| Structure | Common Name | Systematic Name |
| (CH3)2CH- | Isopropyl | 1-Methylethyl |
| (CH3)2CH-CH2– | Isobutyl | 2-Methylpropyl |
| CH3CH2CH(CH3)- | Sec-butyl | 1-Methylpropyl |
| (CH3)3C- | Tert-butyl | 1,1-Dimethylethyl |
| (CH3)3C-CH2– | Neopentyl | 2,2-Dimethylpropyl |
SECOND METHOD
Another method of naming the branching of substituents is-
Count the total number of carbon present in the branch. Then determine the minimum position with which it is connected with the stem chain.
(CH3)2CH- is called 1-methylethyl according to the first method. According to the second method it will be called propan-2-yl
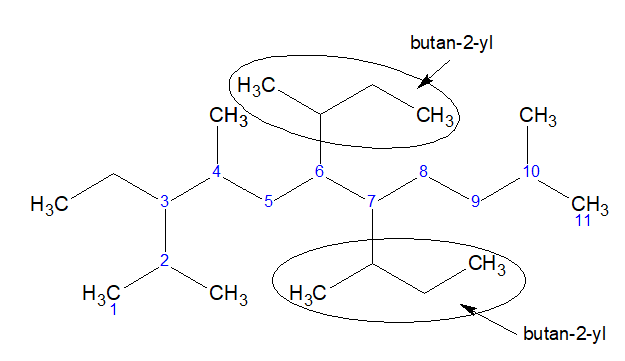
So, the above compound is 6,7-bis(butan-2-yl)-3-ethyl-2,4,10-trimethylundecane
You can also refer to this video.