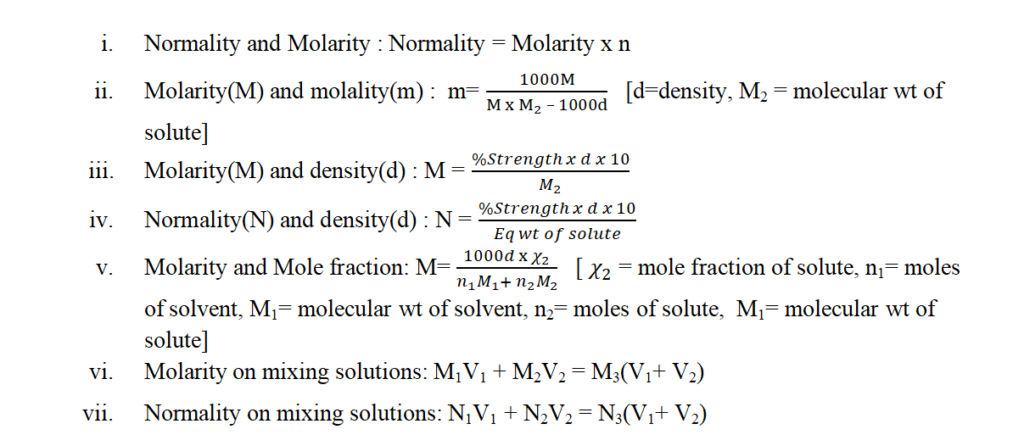
1.Solubility:
It is equal to the amount of solute dissolved in 100 g of the solvent to form a saturated solution at a given temperature.
2.Factors affecting the solubility of a solid in a liquid:
a)Nature of solute and solvent:
“Like dissolves like”. Ionic compound dissolves in polar solvents. Covalent and organic compounds dissolve in non-polar solvents.
| Polar solvents | Non-polar solvents |
| Water | Benzene |
| Liquid ammonia | Chloroform |
| Liquid hydrogen sulphide | Ether |
| Liquid sulphur dioxide | Carbin tetrachloride |
b)Effect of temperature:
- Solubility increases with temperature: NaNO3, KNO3, NaCl, KCl. Dissolution is endothermic.
- Solubility decreases with temperature: Ce2SO4, Li2CO3,Na2CO3.H2O, Dissolution is exothermic.
- Anomalous solubility:NH4NO3, CaCl2.6H2O, Na2SO4.10H2O
c)Pressure:
Does not have any significant effect on solubility.
3.Factors affecting the solubility of a gas in a liquid:
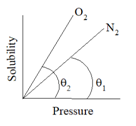
a)Nature of the gas and solvent:
| Solvent | Slightly soluble gas | Highly soluble gas |
| Water | H2, N2, O2 | CO2, NH3, HCl, H2S |
| Ethanol | NH3, H2S | N2, O2, CO2 |
b)Effect of temperature:
With the increase in temperature the solubility of gases decreases.
- The solubility of H2 and inert gases increases slightly with an increase of temperature in hydrocarbon, alcohol, and acetone.
- ln[C2/C1]=∆H/R (1/T1 – 1/T2), C1 , and C2 are the concentration of the solutions at T1 and T2 respectively. ∆H is the heat of solution of 1 mole of the gas.
c)Effect of pressure:
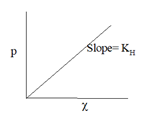
Henry’s Law:
The mass of a gas dissolved in a given volume of the liquid at constant temperature is directly proportional to the pressure of the gas present in equilibrium with the liquid.
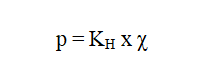
Where, p= partial pressure of gas, χ = mole fraction of the gas in solution,KH = Henry’s constant which depends upon, temperature, nature of the solvent, nature of the gas.Higher the slope (value of K), higher is the solubility of the gas.
Henry’s Law can be applied only when:
- Pressure is low and the temperature should be high.
- The gas should not be highly soluble in water.
- The gas should not react with the solvent.
- The gas should not under any association or dissociation in the solvent.
Applications of Henry’s Law:
- To increase the solubility of CO2 in soda water and aerated drinks, the bottle is sealed under high pressure.
- To avoid bends and also toxic effects of high concentration of nitrogen in the blood, the cylinders used by the scuba divers are filled with air diluted with 11.7% He, 32.1% oxygen, 56.2% nitrogen.
- When air enters the lungs, partial pressure of oxygen being high, haemoglobin combines with oxygen to form oxyhaemoglobin. The partial pressure of oxygen in tissues is low. Hence, oxygen is released from oxyhaemoglobin which is utilised by the cells.
- Anoxia is felt only due to the low concentration of oxygen in the blood and tissues which occurs at high altitudes, where the partial pressure of oxygen is low.
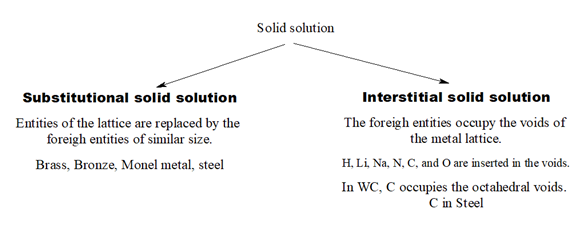
4.Solid solution:
5.Vapour Pressure:
The pressure exerted by the vapours in equilibrium with the liquids at a constant temperature.
Factors effecting Vapour pressure:
- Nature of the liquid: weaker the intermolecular force of attraction, higher the vapour pressure.
- Temperature: With the increase in temperature, vapour pressure increases.
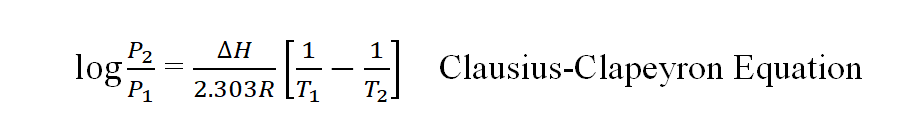
- Liquids having high b.p have low vapour pressure.
- When a non-volatile solute is dissolved in a pure liquid, the vapour pressure of the solution decreases.
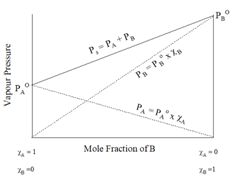
7.Raoult’s Law:
a)Raoult’s Law for volatile solutes:
In a solution, the vapour pressure of a component at a given temperature is equal to the mole fraction of that component in the solution multiplied by the vapour pressure of that component in the pure state.
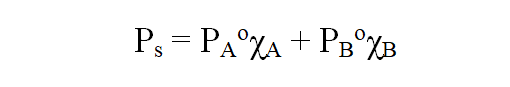
b)Raoult’s Law for non-volatile solutes:
The relative lowering of vapour pressure of a solution containing a non-volatile , non-electrolyte solute is equal to the mole fraction of the solute in the solution.
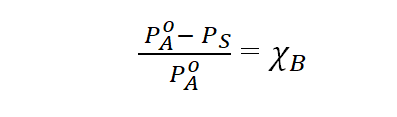
8.Ideal solution:
- A solution that obeys Raoult’s law over a wide range of concentration and temperature.
- For ideal solution, ∆Vmixing = 0, ∆Hmixing = 0
- Example: Benzene + Toluene, n-Hexane + n-Heptane, Ethyl chloride + Ethyl bromide, Bromobenzene + Chlorobenzene, Methanol+Ethanol, Carbon tetrachloride + Silicon tetrachloride, Ethylene bromide + Ethylene iodide.
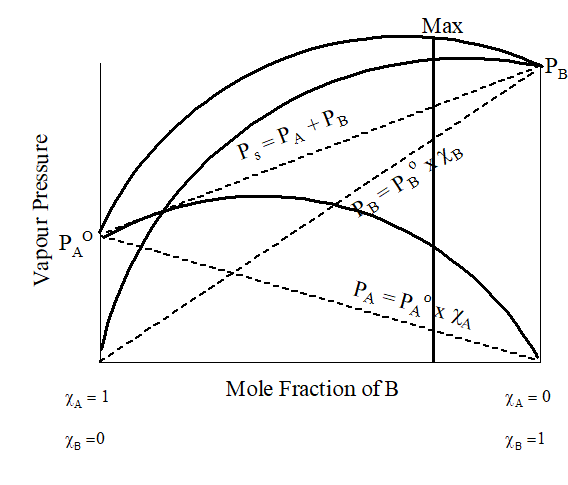
9.Non-Ideal Solution:
| Positive Deviation | Negative Deviation |
| Interaction between the components is less than in the pure components. | Interaction between the components is greater than in the pure components. |
| Boiling point is lower than the components | Boiling point is higher than the components |
| ∆Hmixing > 0 | ∆Hmixing < 0 |
| ∆Vmixing > 0 | ∆Vmixing < 0 |
| PA> PA0.χA and PB> PB0.χB | PA< PA0.χA and PB< PB0.χB |
| Form minimum boiling azeotropes | Form maximum boiling azeotropes |
| Ethanol + Acetone, Benzene + Acetone, Water + Ethanol, Water + methanol, Cyclohexane +Ethanol, CCl4 + C6H6, CCl4 + Toluene, CCl4 + CHCl3, CCl4 + acetone | Chloroform + Acetone, chloroform + benzene , water + HCl, Water + NaCl, Acetic acid + pyridine, Acetone + aniline |
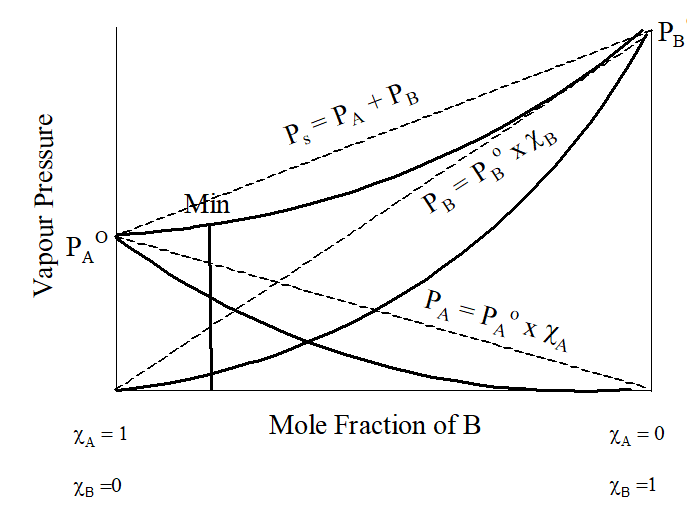
Negative Deviation
10.Azeotropes:
Liquid mixture having, a definite composition and boiling point like a pure liquid.
Positive deviations → Minimum boiling azeotropes. Negative deviations → Maximum boiling azeotropes.
11.Colligative Properties:
Those properties of the solutions which depends only upon the number of solute particles in a given volume of the solvent.
12.Relative lowering of vapour pressure:
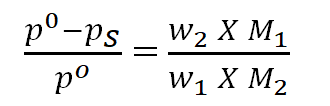
n1, w1, M1 = no of moles, mass in g and molecular mass of the solvents.
n2, w2, M2 = no of moles, mass in g and molecular mass of the solutes.
p0 = vapour pressure of the pure solvent.
pS= vapour pressure of the solution.
- Vapour pressure of a liquid or a solution is not a colligative property but relative lowering of vapour pressure is a colligative property.
- Lowering of vapour pressure is measure by Ostwald-Walker dynamic method or transpiration method.
13.Osmosis:
The spontaneous movement of the solvent molecules either from pure solvent into the solution or from dilute solution into the concentrated solution through a semipermeable membrane.
Osmotic Pressure:
The external pressure that has to be applied on the solution to prevent the osmosis of the solvent into it through a semipermeable membrane.
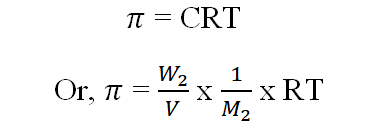
W2= wt of solute,M2= Molecular wt of solute,V= Volume of the solution in L,T=Temperature in K,R=0.0821 L atm
Isotonic Solution:
Two solution having the same osmotic pressure are said to be isotonic to each other at the same temperature.
Reverse osmosis:
When the external pressure is more than the osmotic pressure, solvent molecules moves from the most concentrated solution to the less concentrated solution. This process is called reverse osmosis.
This process is used to prepare slat(Desalination), purifying drinking water(RO system)
14.Elevation of boiling point:
When a non-volatile, non-electrolyte solute is added to a pure solvent, the boiling point of the solution increases as the vapour pressure decreases.
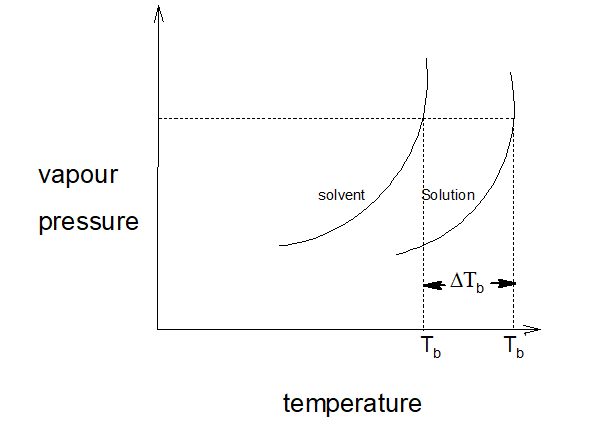
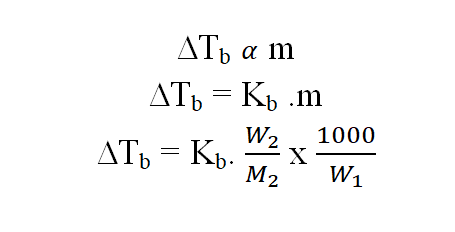
w1, M1 = mass in g and molecular mass of the solvents. w2, M2 = mass in g and molecular mass of the solutes. ∆Tb = Increase in boiling point. Kb = molal elevation constant or molal ebullioscopic constant of the medium.
- Relation between molal elevation constant or molal ebullioscopic constant and molar enthalpy of vaporisation:
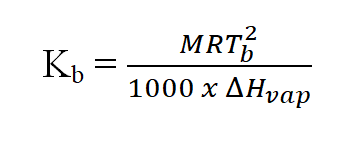
M= Molar mass of the solvent in g/mol, T= Boiling point of the solvent, ∆Hvap= Molar enthalpy of vaporisation (molar latent heat of vaporisation) of the solvent.
15.Depression of freezing point:
When a non-volatile, non-electrolyte solute is added to a pure solvent, freezing point of the solution decreases.
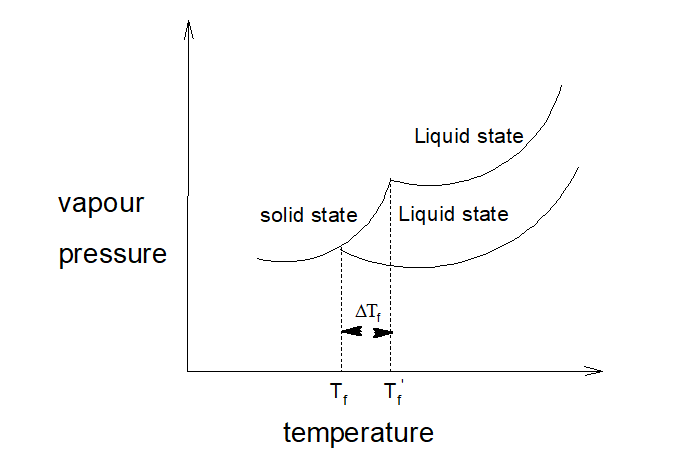
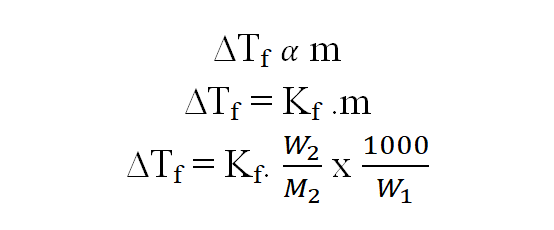
w1, M1 = mass in g and molecular mass of the solvents. w2, M2 = mass in g and molecular mass of the solutes. ∆Tf = Decrease in freezing point. Kf = molal depression constant or molal cryoscopic constant of the medium.
- Relation between molal depression constant or molal cryoscopic constant and molar enthalpy of fusion:
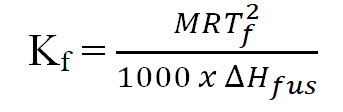
M= Molar mass of the solvent in g/mol, T= Freezing point of the solvent, ∆Hvap= Molar enthalpy of v fusion (molar latent heat of fusion) of the solvent.
16.Expressing concentration of solutions:
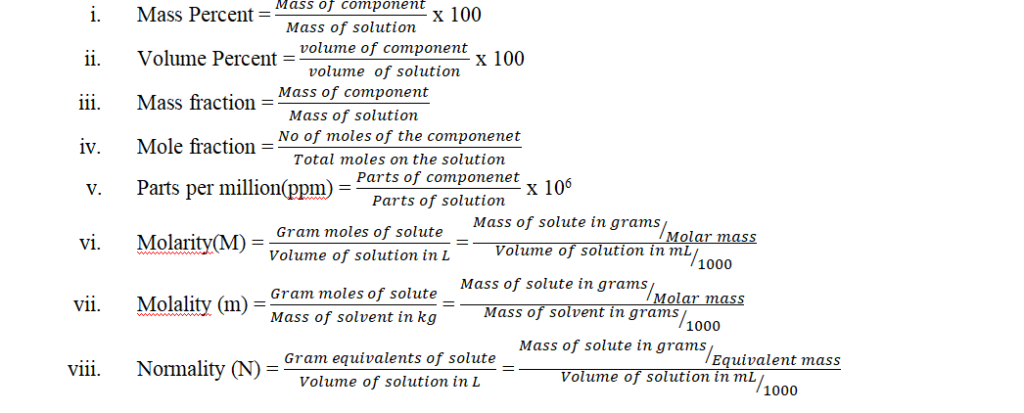
Relation between: