What is adsorption?
The phenomenon of attracting and retaining the molecules of a substance on the surface of a liquid or a solid resulting into a higher concentration of the molecule on the surface is called adsorption.
What is adsorbate and adsorbent?
The substance adsorbed on the surface is called the adsorbate and the substance on which it is adsorbed is called adsorbent.
What is desorption?
The removal of the adsorbed substance from the surface is called desorption.
What is occlusion?
The adsorption of gases on the surface of metals is called occlusion.
What is the main cause of adsorption?
In case of liquid and solid adsorbents, the adsorption is due to the unbalanced forces acting inwards. In case of metals, it is due to the presence of free valencies on metal surface.
How ∆H and ∆G changes during adsorption?
As molecules are held on the surface , entropy decreases, ∆S=-ve. As adsorption is a spontaneous process, ∆G = -ve. As ∆G = ∆H-T∆S , thus ∆H is –ve.
What is adsorption equilibrium?
As adsorption proceeds, ∆H keeps on decreasing and T∆S keeps on increasing till ultimately ∆H becomes equal to T∆S so that ∆G = 0. This state is called adsorption equilibrium.
What is sorption?
When adsorption and absortion take place simultaneously the process is called sorption.
Difference between adsorption and absorption?
| Adsorption | Absorption |
| It is a surface phenomenon which occurs only at the surface of the adsorbent. | It is abulk phenomenon. |
| Concentration on the surface of adsorbent is different from that in the bulk. | Concentration is same throughout the material. |
| Its rate is high in the beginning and then decreases till equilibrium is attained. | Its rate remains same throughout the process. |
What is positive and negative adsorption?
When the concentration of the adsorbate is more on the surface of the adsorbent than in the bulk, it is called positive adsorption.
If the concentration of the adsorbate increases in the bulk after adsorption, it is called negative adsorption.
Mention some example of adsorption and absorption.
- Silica gel adsorbs water vapor, anhydrous CaCl2 absorb it.
- Ammonia is adsorbed in charcoal, ammonia is absorbed in water.
- Dyes get adsorbed and absorbed,i.e., sorption takes place.
Mention the factors which affect the adsorption of gases by solids.
- Nature and surface area of the adsorbent: With the increase in surface area of the adsorbent, the volume of gas adsorbed increases.
- Nature of the gas: More easily liquefiable or water-soluble gas( high critical temperature), more readily it is adsorbed.
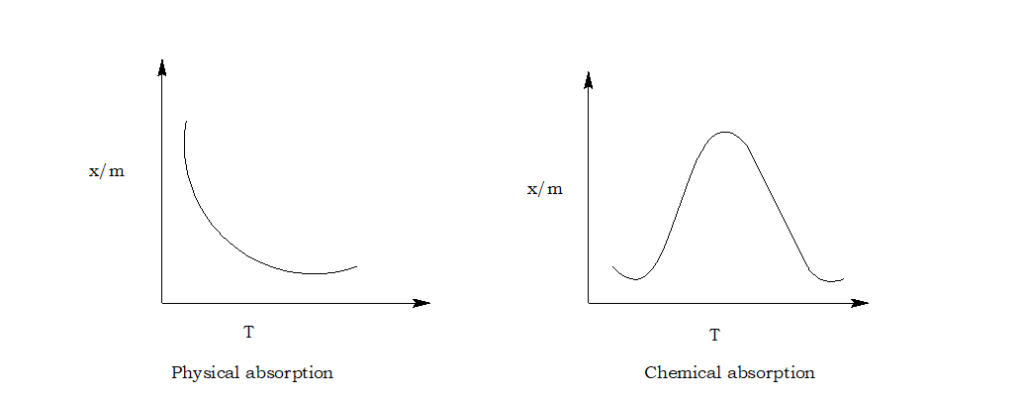
- Temperature: Adsorption decreases with the increase in temperature.
- Pressure: At constant temperature, adsorption of gas increases with the increase in pressure.
- Activation of the solid adsorbent: By increasing the surface area of the adsorbent, by mechanical rubbing, chemical action, by subdividing the adsorption into smaller pieces, its adsorbing power increases.
Differentiate between physical and chemical adsorption.
| Characteristics | Physical adsorption | Chemical adsoirtion |
| Type of bonding | Van der waal | Similar to chemical bond |
| Heat absorption | Low ( 20 to 40 kJ) | High (40 to 400kJ) |
| Formation of compound | No compound formed | Surface compounds are formed |
| Reversibility | Reversible | Irreversible |
| Activation energy | Not required | Required |
| Temperature favours | Low | High |
| Specificity | Not specific | Specific |
| Relation between gas absorbed and ease of liquefaction | Directly related | No such correlation |
| Layer formation | Forms multimolecular layer | Forms unimolecular layer |
What is adsorption isotherm?
The curve obtained by plotting the amount of gas adsorbed against gas pressure at a constant temperature is called adsorption isotherm.
What is Freundlich isotherm?
If x/m is the amount of gas absorbed per gram of the absorbent at the pressure p, k and n are constants which depends upon the nature of gas and adsorbent at a particular temperature.
Then,



This isotherm fails if the concentration or pressure is high.
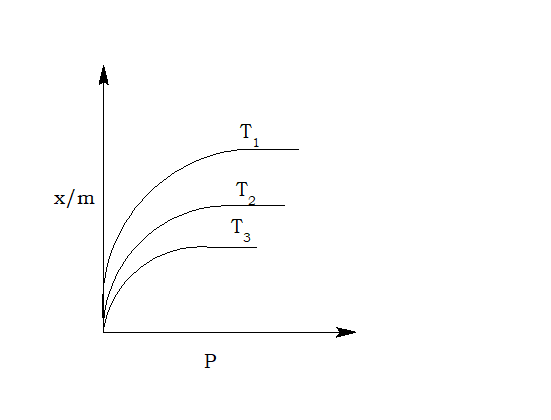
What is adsorption isobar?
A graph drawn between the amount of the gas adsorbed per gram of the adsorbent and temperature at a constant pressure of adsorbate gas is known as adsorption isobar.
- Dispersed phase:
- In a colloidal system, the component which remains in a small amount and which is distributed in the dispersed medium in the form of colloidal particles (1 to 100nm) is known as the dispersed phase.
- Dispersion medium:
- The medium in which the colloidal particles are dispersed is called the dispersion medium.
- Colloids:
- When particles of diameter 10-7 m to 10-9 m of an insoluble substance are kept in suspension in solvent where they move freely, do not form a clear solution but form a translucent heterogeneous mixture from which the particle cannot be removed by passing through parchment paper are called colloids.
| Properties | Suspension | Colloids | True solution |
| Size of particles | More than 100nm | Between 1 to 100nm | Less than 1nm |
| Visibility | Visible by naked eye and microscope | Not visible by naked eye or microscope but by ultra microscope | Not visible even by ultra microscope |
| Nature | Heterogeneous | Heterogeneous | Homogeneous |
| Electrical property | Particles and the whole system are neutral | Colloid particles are either positively or negatively charged | Neutral |
| Settling of particles | Precipitates under the force of gravity | Coagulates by the addition of electrolytes | Cannot be coagulated |
| Colour | Colour depends upon the particles | Colour depends upon the size of the colloid particles | Colour depends upon the soluble ions |
| Tyndall Effect | Light cannot pass | Exhibits Tyndall Effect | Does not exhibit Tyndall Effect |
| Passing through filter and parchment paper | Cannot pass through filter paper or parchment paper | May pass through filter paper but not through parchment paper | Can pass through filter and parchment paper |
| Diffusion | Does not diffuse | Diffuse slowly | Diffuses quickly |
| Dialysis | Cannot pass through a dialyzer | Cannot pass through a dialyzer | Can pass through a dialyzer |
| Brownian movement | May occur | Occur | Do not exhibit |
Classification of colloids:
A.Based on physical state :
| Dispersed phase | Dispersion Medium | Typical name | Examples |
| Solid | Solid | Solid solution | Coloured glass, gem stones |
| Solid | Liquid | Solution | Paints, gold sols,muddy water |
| Solid | Gas | Solid aerosol | Smoke, dust particles in air |
| Liquid | Solid | Gel | Jelly, cheese, butter |
| Liquid | Liquid | Emulsion | Milk, hair cream |
| Liquid | Gas | Liquid aerosol | Fog, cloud |
| Gas | Solid | Solid foam | Foam rubber, pumice stone |
| Gas | Liquid | Foam | Whipped cream, froth |
B. Based on the nature of interactions:
| Properties | Lyophobic sols | Lyophilic sols |
| Preparation | By special methods | By direct mixing |
| Size of particles | Each colloid particle consists of atoms or molecules | Each colloidally dissolved unit in liquid is a single macro molecule |
| Surface tension | Similar to that of dispersion medium | Lower than that of dispersion medium |
| Viscosity | Does not differ much from that of the medium | Much higher than that of the medium |
| Nature | Irreversible | Reversible |
| Stabilization | Less stable | Thermodynamically stable |
| Tyndall effect | Exhibits | Does not exhibits |
| Action of electrolysis | Precipitation is caused | Precipitation is not caused |
| Action of electrical field | Particles migrates | Particles may or may not migrates |
| Colligative properties | Insignificantly small | Well marked |
| Solvation | Not solvated | generally solvated |
| Charge | Particles carry +ve or –ve | Particles carry a little or no charge. |
C. Based on the type of particles of the dispersed phase:
| Multi molecular colloids | Macro molecular colloids | Associated Colloids or Micelles |
| Formed by the aggregation of a large no of atoms or molecules having diameters less than 1nm | Such type of colloidal particles are themselves big size molecules having dimensions of colloid particles | Formed by the aggregation of a large no of ions in concentrated solutions. |
| Ex: Sols of Ag,Au,S | Ex: Starch, Proteins, | Ex: Soap, Detergents |
| Low Molecular masses | High | High |
| Weak VW bond | Strong VW bond | Strong VW bond |
Preparation of colloids:
Lyophilic colloids:
Prepared simply by dissolving glue, gelatin, gum, the starch in a suitable solvent in hot condition.
Lyophobic Colloids:
a) Dispersion method-
- Mechanical dispersion
- Electro disintegration
- Peptization
b) Condensation method-
- Chemical reaction
- Exchange of solvent
- Excessive cooling
- Condensing vapors in solvent
Important definition:
- Sols: Colloidal system of solid in a liquid.
- Gels: Colloidal system of liquid in solid.
- Emulsion: Colloidal system of liquid in liquid.
| Dispersion medium | Name of sol |
| Water | Aqua or hydra sol |
| Alcohol | Alcosol |
| Benzene | Benzosol |
| Gases | Aerosol |
- Micelle: The aggregated particles formed due to the association of colloids in the medium.
- Critical Micelle Concentration (CMC): Formation of micelle takes place above this particular concentration.
- Kraft temperature: Formation of micelle takes place above this particular temperature.
- Dialysis: Method of separation of a crystalloid from a colloid by passing the mixture of the two, through a membrane of parchment paper or fish bladder or collodion membrane.
- Electrophorosis: Movement of colloidal particles under the influence of the electric field.
- Electro osmosis: The movement of dispersion medium under the influence of an electric field keeping the disperse phase stationery with the help of a suitable membrane .
- Electrokinetic phenomenon: When a liquid is forced through a capillary tube or through a porous pot, a potential difference is set up. This is known as streaming potential. Again if solid particles are made to pass through a liquid by sedimentation, a potential difference is set up which is called sedimentation potential. These two effects are collectively called electrokinetic phenomena.
- Tyndall effect: Scattering of light by the colloidal particles present in a colloidal sol.
- Coagulation: Process of aggregation of the colloidal particles so as to bring them into large-sized particles which ultimately settle down as precipitate. If the precipitated colloids float on the medium instead of settling down, it is called flocculation.
- Schulze Hardy Valency Rule:
- In the coagulation of colloids, the ions carrying opposite charges to that of the colloidal particles are most effective.
- The higher the valency of the added ion, the greater is the effective power of the coagulation of the electrolyte and smaller is the amount of electrolyte required to coagulate.
- Al3+> Mg2+ > Na+
- [Fe(CN)6]4-> PO43-> SO42->Cl–
- The influence of the valency of the added ions is in GP. i.e, 1:m:m2
- Flocculation Value: It may be defined as the min conc (in millimoles per L) of an electrolyte required to cause the coagulation of a sol. Smaller its value, greater is its coagulating property.
- Protection of lyophobic colloids: The process of getting extra stability of the lyophobic sols on the addition of lyophilic sols to them.
- Gold no: Gold no of a protective colloid is defined as the min weight in milligrams of dry material from which lyophilic sol is prepared, which when added to 10mL of a standard gold sol is just sufficient to prevent the color change from red to blue on the addition of 1mL of 10% solution of NaCl.
- Iso-electric point: For a lyophilic sol there is a pH when it moves to neither of the electrodes on applying electric fields. This pH is called the iso-electric point of that colloid. At the iso-electric point, the sol behaves as an electrically neutral species.















