Which gas Decolourises potassium permanganate (KMnO4) solution?
(a) Sulphur dioxide
(b) Ammonia
(c) Hydrogen chloride
(d) Carbon dioxide
Ans: Sulphur dioxide
The Chemistry Behind the Decolorization
Potassium permanganate (KMnO₄) is a strong oxidizing agent, meaning it readily accepts electrons. In its usual state, the permanganate ion (MnO₄⁻) imparts a deep purple color to the solution. Sulphur dioxide (SO₂) acts as a reducing agent, meaning it readily donates electrons.
The reaction between SO₂ and KMnO₄ in an acidic medium can be represented by the following simplified ionic equation:
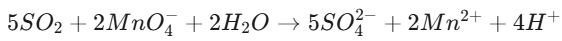
- Oxidation: SO₂ is oxidized to sulfate ions (SO₄²⁻).
- Reduction: The purple permanganate ion (MnO₄⁻) is reduced to the nearly colorless manganese(II) ion (Mn²⁺).
This reduction process is what causes the purple color of the KMnO₄ solution to disappear.
Why the Other Options Are Incorrect
- (b) Ammonia (NH₃): Ammonia can react with KMnO₄, but the reaction is complex and doesn’t simply result in decolorization. It can lead to the formation of manganese dioxide (MnO₂) which creates a brown precipitate, depending on reaction conditions.
- (c) Hydrogen chloride (HCl): Hydrogen chloride in its gaseous form will not decolourise potassium permanganate. When it is dissolved in water, forming hydrochloric acid, then it can react with potassium permanganate, but it is not the most efficient gas to do so.
- (d) Carbon dioxide (CO₂): Carbon dioxide is relatively inert in this context. It does not readily react with KMnO₄ to cause decolorization.
Practical Applications and Observations
- This reaction is often used as a test for the presence of sulphur dioxide.
- The decolorization is more rapid in an acidic medium.
- The reaction is a redox reaction.
- The reaction is used in some industrial processes to remove sulphur dioxide from waste gases.















